Intraperitoneal hemorrhage due to segmental arterial mediolysis associated with cerebral vasospasm after subarachnoid hemorrhage
Abstract
A man in his 50s with no significant past medical history developed subarachnoid hemorrhage due to ruptured left middle cerebral artery aneurysm. On the ninth hospital day, he had a ruptured visceral aneurysm with segmental arterial mediolysis, and we successfully treated with transarterial embolization using metallic coils.
1 INTRODUCTION
Segmental arterial mediolysis (SAM) is a noninflammatory, nonatherosclerotic disease that causes segmental lysis of the outer arterial media.1 It can result in the separation of the media from the adventitia leading to dissecting aneurysms.1 Generally, an aneurysm forms in large abdominal aortic branches, and its rupture causes intra-abdominal hemorrhage.2 Cerebral arteries can be also involved, but SAM is not yet well known in the neurological field. In rare cases, SAM has been reported to occur in conjunction with subarachnoid hemorrhage (SAH). We report a case of SAM-induced intra-abdominal bleeding during the cerebral spasm after SAH, which was successfully treated with coil embolization of an intraperitoneal aneurysm.
2 CASE HISTORY
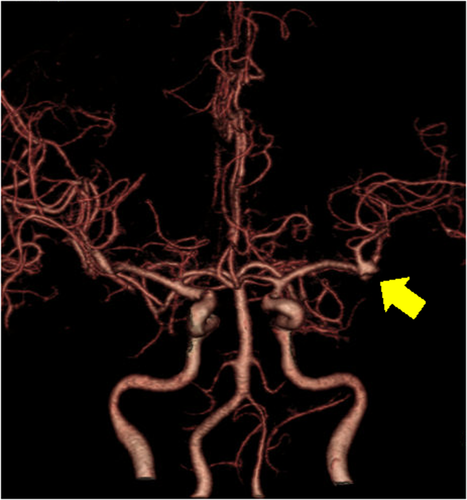
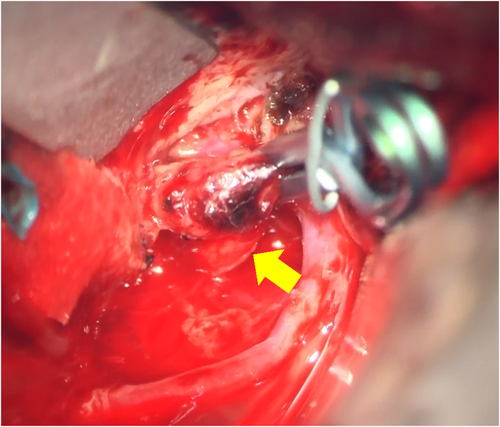
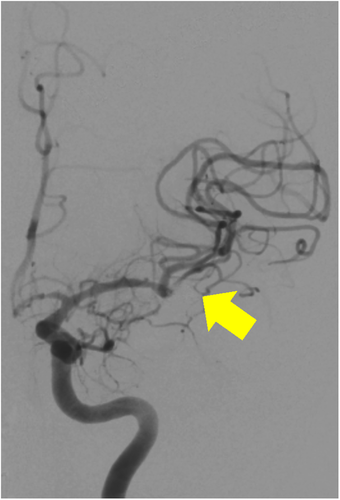
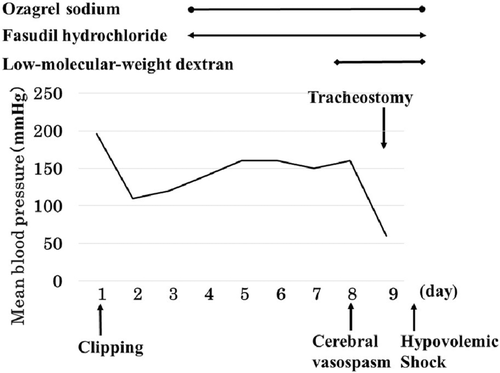
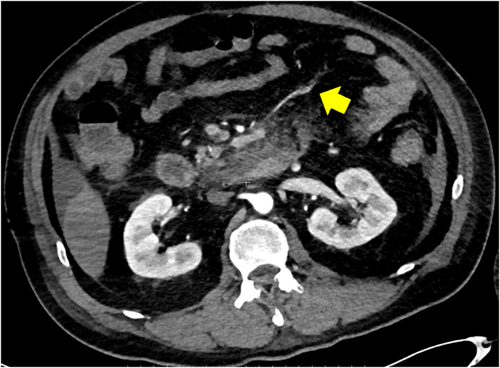
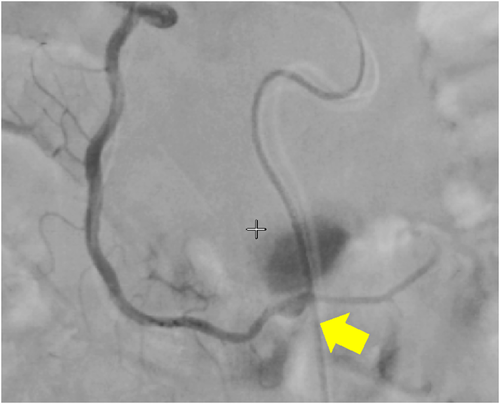
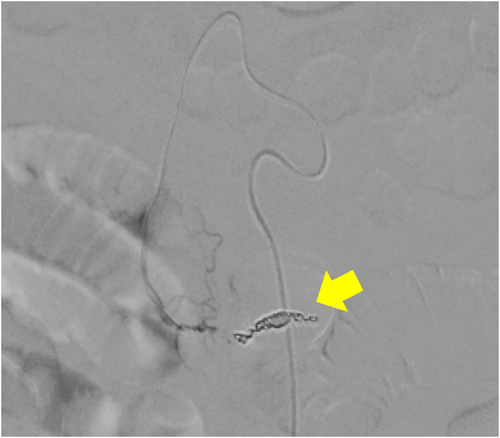
A 59-year-old man with no significant past medical history suddenly lost consciousness after the onset of a severe headache. He presented to the emergency department of our hospital. On admission, his Glasgow Coma Scale was E3V2M5. A clinical diagnosis of grade IV was made based on the World Federation of Neurological Surgeons [WFNS] scale. Computed tomography (CT) scan showed SAH from the basal cistern to the left Sylvian fissure (Fisher group 3). Three-dimensional CT angiography revealed an aneurysm (6.7 × 6.2 × 4.9 mm) with blebs in the middle cerebral artery (M2) bifurcation of the left MCA. (Figure 1) Non-contrast-enhanced CT of the chest and abdomen for screening showed no abnormalities. We diagnosed SAH due to ruptured left MCA aneurysm and performed clipping for the aneurysm on the same day. External decompression was also added because of the elevated cerebral pressure. Intraoperative findings indicated the aneurysm was a saccular appearance. (Figure 2) To prevent cerebral vasospasm, spinal drainage was started postoperatively, and the administration of ozagrel sodium 80 mg/day and fasudil hydrochloride hydrate 30 mg × 3/day was started. Eight days after onset, cerebral angiography showed narrowing at the left M2 due to cerebral vasospasm and delayed blood flow in peripheral vessels. (Figure 3) Therefore, low-molecular-weight dextran was added to maintain cerebral blood flow, and blood pressure was maintained at a higher level. (Figure 4) On the ninth hospital day, when we performed tracheostomy for respiratory management because of prolonged disturbance of consciousness, he suddenly lapsed into a hypovolemic shock state. The head CT showed no new intracranial hemorrhage or infarction. However, non-contrast and contrast-enhanced CT of the chest and abdomen showed bloody ascites and abnormal dilatation of the superior mesenteric artery and anterior superior pancreatoduodenal artery (ASPDA), suspected intraperitoneal hemorrhage from the same site. (Figure 5) We determined that the patient was in hemorrhagic shock due to abdominal hemorrhage. He was administered catecholamine, blood transfusion, and insertion of an aortic balloon pumping to stabilize his circulation. An emergency abdominal angiography revealed the beaded appearance of the ASPDA. (Figure 6) The ruptured ASPDA pseudoaneurysm was successfully treated by transarterial coil embolization. (Figure 7) Immunological tests performed to investigate the cause of the disease showed that the level of proteinase-3 antineutrophil cytoplasmic antibody (PR3ANCA) was mildly elevated. Antinuclear antibodies, anticardiolipin antibodies, and myeloperoxidase-anti-neutrophil cytoplasmic antibodies (MPOANCA) were negative, and complement titers, C3 and C4, were not elevated. Ten days after the Onset of SAH, CT revealed severe left MCA territory infarction caused by cerebral vasospasm and we performed conservative treatment for the patient. On Day 44, the patient had good cerebral pressure control and underwent cranioplasty. He was transferred to a rehabilitation hospital on Day 77 and was discharged home with a modified Rankin Scale 3.
3 DISCUSSION
SAM was first reported by Slavin in 1976, based on the pathological examination of cases of intra-abdominal hemorrhage caused by abdominal aneurysms.3 There are two main types of arteries: the elastic arteries, and the muscular arteries. Elastic arteries, such as the aorta and common carotid artery, consist of elastic tissues in the tunica media. Muscular arteries, such as the cerebral arteries and visceral arteries, contain smooth muscle cells in the tunica media.4 SAM occurs in muscular arteries because of segmental lysis of the arterial tunica media smooth muscle cells.2 It often shows findings of a spindle aneurysm or dissection.5-10 The definitive diagnosis of SAM is based on pathological findings, and the clinical diagnosis depends on the exclusion of other vasculitis and findings of beaded vessel irregularities, dissection, or aneurysm on angiography. Specifically, the criteria are as follows: (1) middle-aged and older patients, (2) exclusion of underlying diseases such as inflammatory changes or atherosclerotic changes, (3) sudden onset of intra-abdominal bleeding, and (4) presence of bead-like changes in blood vessels on angiography.11 Differential diseases include atherosclerotic disease, systemic vasculitis (Behcet's disease, polyarteritis nodosa), fibromuscular dysplasia (FMD), Ehlers–Danlos disease type IV, mycotic aneurysms, cystic medial necrosis, and Marfan syndrome.1
In this case, the patient was a middle-aged man in his 50s with no history of collagen disease or other inflammatory or atherosclerotic diseases. His laboratory blood samples showed no elevated inflammatory response, and MPOANCA and complement titers were normal. Only PR3ANCA was elevated in immunological tests, which required differentiation from granulomatous polyangiitis. It was not characteristic because of no findings of upper respiratory tract symptoms, pulmonary symptoms, or nephritis. On the ninth hospital day, the patient developed sudden intra-abdominal hemorrhage, and abdominal angiography showed beaded vasodilatation confined to the ASPDA. Based on the above, SAM was diagnosed because it met the criteria for clinical diagnosis.
The etiology of SAM is still unclear. However, some authors believe that vasoactive substances such as norepinephrine are involved and cause vasoconstriction.12, 13 When SAH occurs, the activation of the sympathetic nervous system causes a surge of catecholamines, which stimulate α1 receptors widely distributed in the smooth muscle cells of the tunica media.12 Because systemic norepinephrine increases approximately threefold within 48 hours of onset, an intra-abdominal aneurysm may have formed at an early stage after the SAH onset in this case.14 The blood pressure was maintained at a high level during the management of cerebral vasospasm and the blood pressure further increased due to stimulation associated with tracheostomy. Therefore, it may have contributed to the aneurysm rupture.
Including this case, 14 cases of intraperitoneal hemorrhage due to SAM after SAH have been reported.2, 5-10, 15-20 (Table 1) There are many reports from Japan. This is because of the high incidence of SAH in Japan compared with the worldwide.21 Intracranial cerebral aneurysms included 6 dissecting aneurysms, 2 blistering aneurysms, and 6 saccular aneurysms. Including this case, 11 patients had intraperitoneal hemorrhage during the cerebral vasospasm period after SAH. Cerebral vasospasm often begins 3–4 days after onset, peaks at 7–10 days, and lasts until about 14–21 days.22 This is the period when SAM may occur. The blood pressure is often maintained high, and antiplatelet medications are used to manage cerebral vasospasm. It may contribute to intraperitoneal hemorrhage.
| No. | Author, year | Age, sex | Intracranial aneurysm Site | Aneurysm type | WFNS grade | Treatment of intracranial aneurysm | Symptoms before intraperitoneal hemorrhage | Visceral aneurysm | Duration of intraperitoneal hemorrhage after SAH (days) | Cerebral infraction due to cerebral vasospasm | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fuse et al. (1996)15 | 56, F | Left ICA | Saccular | II | Clipping | Abdominal pain | Gastroepiploic, gastric artery | 16 | N.A. | No deficits |
| 2 | Sakata et al. (2002)5 | 48, M | RightVA, Left ICA | Dissection | V | None | N.A. | Superior mesenteric artery, left external iliac artery | N.A. | N.A. | mRS6 |
| 3 | Stetler et al. (2012)16 | 59, F | Right IC-PC | Saccular | III | IVR | Abdominal pain | Hepatic artery | 3 | None | mRS0 |
| 4 | Shinoda et al. (2016)6 | 47, M | Left VA | Fusiform | V | IVR | Abdominal pain | Middle colic artery | 8 | N.A. | mRS3 |
| 5 | Welch et al. (2017)7 | 61, M | PSA | Fusiform | N.A. | IVR | Anemia | Splenic artery | 0 | N.A. | No deficits |
| 6 | Hellstern et al. (2017)8 | 30, M | Both ICA | Dissection | IV | IVR | Shock | Splenic artery | 0 | N.A. | Barthel index of 90 |
| 7 | Hayashi et al. (2018)2 | 49, F | Left ICA | Saccular | II | Clipping | Abdominal pain | Splenic artery | 4 | Left temporal lobe infraction | mRS0 |
| 8 | Isaji et al. (2018)9 | 45, M | Right VA | Dissection | II | IVR | Shock | Superior mesenteric artery | 8 | None | mRS0 |
| 9 | Ohara et al. (2019)10 | 82, F | Left VA-PICA | Fusiform | II | IVR | Shock | Hepatic artery | 14 | Cerebellar infraction | mRS5 |
| 10 | Inazuka et al. (2019)17 | 77, F | Right ICA | BBA | II | Clipping | Anemia | Celiac artery, splenic artery | 8 | None | mRS6 |
| 11 | Hori et al. (2020)18 | 67, F | Right IC-PC | Saccular | II | Clipping | Abdominal pain | Splenic artery | 14 | Right frontal lobe infraction | mRS1 |
| 12 | Tanaka et al. (2020)19 | 78, M | Right ICA | BBA | III | Trapping with bypass | Shock | Posterior inferior pancreaticoduodenal artery | 12 | Right ICA territory infraction | mRS6 |
| 13 | Ota et al. (2020)20 | 54, M | Acom | Saccular | IIorIII | Clipping | Shock | Right gastroepiploic artery | 6 | None | mRS2 |
| 14 | Present case (2021) | 59, M | Left MCA | Saccular | IV | Clipping | Shock | Anterior superior pancreatoduodenal artery | 9 | Left MCA territory infraction | mRS3 |
- Abbreviations: Acom, anterior communicating artery; BBA, blood blister-like aneurysm; F, female; ICA, internal carotid artery; IC-PC, internal carotid-posterior communicating artery; IVR, interventional radiology; M, male; MCA, middle cerebral artery; mRS, modified Rankin Scale; NA, not available; PICA, posterior inferior cerebellar artery; PSA, posterior spinal artery; SAH, subarachnoid hemorrhage; VA, vertebral artery; WFNS, World Federation of Neurological Surgeons.
The cerebral infarction caused by cerebral vasospasm was reported in 5 cases. SAH with cerebral vasospasm may be prone to intra-abdominal vasospasm as well, which may lead to aneurysm formation. Therefore, SAM should be considered when cerebral vasospasm occurs after SAH. In many cases, patients had a good level of consciousness before the intra-abdominal hemorrhage and were able to report abdominal symptoms. Therefore, they suspected abdominal disease from the beginning and were able to examine the patients. On the contrary, our patient was unconsciousness due to severe SAH, making it difficult to differentiate the cause of shock. Recognizing SAM can lead to early treatment and save their lives.
4 CONCLUSION
SAH with cerebral vasospasm may be associated with SAM, suggesting that SAM-induced intra-abdominal hemorrhage may occur during cerebral vasospasm. SAM should be considered in the treatment of SAH in patients with impaired consciousness and unable to complain of abdominal pain.
AUTHOR CONTRIBUTIONS
CM wrote and drafted the manuscript. CM, YF, ST, KK, NH, and NS helped draft the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
None declared.
ETHICAL APPROVAL
All subjects enrolled in this research have given their informed consent, which has been approved by the institutional committee on human and/or animal research, and this protocol has been found acceptable by them.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
All data included in this report are accurate to the best of our knowledge. We will make available data (images and reports) upon request.