COVID-19 vaccine-associated myositis – a case report
Abstract
Myositis is one of the uncommon adverse events following COVID-19 vaccination, and its mechanism is still unclear. A strong clinical suspicion and further evaluation are important not only for early diagnosis and management but also for better understanding of the unprecedented effects of this novel vaccine. We present a case of myositis following the first dose of the ChAdOx1 nCoV-19 Corona Virus Vaccine, evidenced by serology and MRI.
1 INTRODUCTION
The most commonly reported adverse events following COVID -19 vaccinations are pain and swelling at the injection site, fever, myalgia, headache, and chills.1 Apart from these, there have also been reports of few rare and life-threatening adverse reactions like thrombosis with thrombocytopenia syndrome,2 Guillain-Barré syndrome,3 vasculitis,4 autoimmune hepatitis,5 inflammatory myopathies,4 and myocarditis,6 which are now thought to be caused by immune-mediated mechanisms. With 6.08 million doses of COVID-19 vaccine being administered daily (as of 5 July 2022),7 the reporting of such rare adverse events is essential not only for a better understanding of its pathogenesis but also to aid clinicians in promptly recognizing the symptoms and for effective management.
2 CASE REPORT
A 53-year-old man presented himself with severe pain and soreness in his left upper arm and generalized myalgia. The pain started 2 days after the first dose of the ChAdOx1 nCoV-19 Corona Virus vaccine (COVISHIELD) into his left deltoid muscle. The pain initially developed in the left upper arm, followed by the right upper arm and bilateral calf muscles (left more than right). The pain gradually increased, restricting his daily activities, following which he presented himself to our hospital, on the 11th day of vaccination. He had difficulty walking and moving his left arm at the time of presentation. He also reported generalized muscle weakness, more pronounced in the proximal upper and lower limbs than the distal ones. The patient was healthy and asymptomatic prior to the vaccination. There was no history of heavy manual labour or vigorous exercise before the onset of symptoms. There was no malaise or fever. There was no history of any skin rash, breathlessness, dry cough, or joint pain. He was non-diabetic and non-hypertensive, with no history of any connective tissue disorders or allergic reactions to drugs or vaccines. There has been no evidence of COVID-19 infection in the past.
On local examination, there was no swelling or erythema. There was tenderness over the left deltoid muscle and bilateral calf muscles, more so on the left side. The power in the proximal muscle groups of the left upper limb was 3/5 and that of the distal was 4/5, whereas for the proximal and distal lower limb muscle groups on the left side, the power was 3/5. Both proximal and distal muscle groups of the right upper and lower limbs showed power of 5/5 (assessed by the Medical Research Council Scale). Sensory examination was normal. Reflexes were preserved.
Serologic testing yielded mildly increased serum creatine kinase (187 U/L, reference range 40–171 U/L) and alanine transaminase (50 U/L, reference range 10–40 U/L) concentrations. Serum bilirubin, aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and C-reactive protein levels were normal. Urine analysis excluded myoglobinuria.
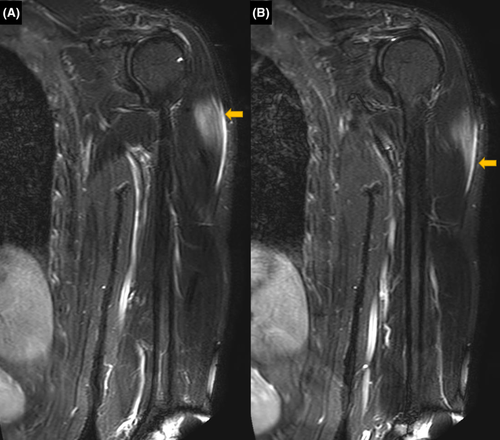
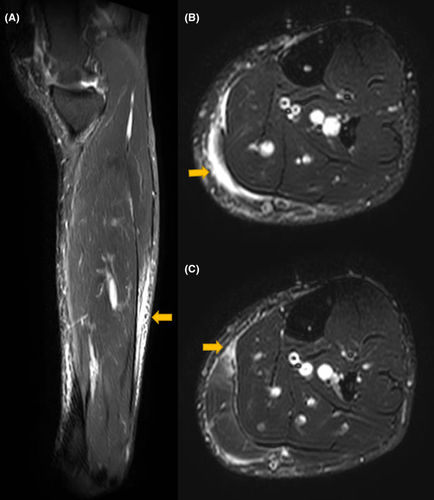
Suspecting skeletal muscle damage, MRI of all the limbs was suggested. On MRI, edema was observed in the left deltoid muscle with a thin layer of subfascial fluid adjacent to the muscle belly (Figure 1). Deltoid muscle architecture was preserved. Subtle edema was also noted in the medial head of the left gastrocnemius muscle, with the edema fluid tracking along the intermuscular plane between the gastrocnemius and soleus (Figure 2). No collection or abscess was seen. MRI of the right arm and leg was normal. Based on the findings, a diagnosis of COVID-19 vaccine-associated myositis was made. EMG, muscle biopsy, and immunological workup could not be carried out as the patient did not give consent.
The patient was advised to rest and take nonsteroidal anti-inflammatory drugs (NSAIDs) for 1 week. Steroids or IVIg were not given, as the symptoms were relatively mild, and the muscle enzymes were only mildly elevated. On the first follow-up after 1 week (18th day of vaccination), the pain and tenderness had reduced considerably. The power in the left upper and lower limb muscle groups was 5/5. However, the difficulty in walking persisted. Repeat serum creatine kinase after 5 weeks (45th day of vaccination) was normal (121 U/L, reference range 40–171 U/L). A repeat MRI was not carried out, as the patient significantly improved after initial management.
3 DISCUSSION
ChAdOx1 nCoV-19 is a recombinant, replication-deficient chimpanzee adenovirus vector which encodes the SARS-CoV-2 Spike (S) glycoprotein. When administered, it causes the genetic material of part of the coronavirus to be expressed, thereby stimulating neutralizing antibody and cellular immune responses.
Autoimmunity associated with vaccines can be attributed to the cross-reactivity between antigens or to the effect of adjuvants.8 ASIA (Autoimmune/inflammatory Syndrome Induced by Adjuvants) is a complex ocurrence referring to the autoimmune manifestations that appear to be caused by adjuvants and may be related to specific HLA phenotypes, the development of autoantibodies, or even evolve into a rheumatological disorder.9 ASIA is characterized by inflammatory musculoskeletal, neurocognitive, and/or constitutional symptoms on exposure to an external stimulus and improves when the stimulus is withdrawn.9 Cases of autoimmune thyroid disease including subacute thyroiditis, Hashimoto's thyroiditis, and Graves' disease have been reported after the first and second doses of COVID-19 vaccines.10 Few of the other reported cases of ASIA following COVID-19 vaccines include optical neuromyelitis, transverse myelitis, autoimmune encephalitis, sensory neuropathy, and polyarthritis autoimmune rheumatoid arthritis.11 K Gupta et al.12 recently reported a case of inflammatory myositis in a 46-year-old female, following the second dose of Covishield. The patient presented with low-grade fever and joint pain, and laboratory tests revealed elevated AST, ALT, LDH, and inflammatory markers. CT chest showed features of interstitial lung disease. MRI showed features of inflammation in bilateral thigh muscles. CK was elevated, and the myositis profile was positive for anti-Jo-1 and anti-Ro-52 antibodies.
It is known that COVID-19 can lead to myositis and rhabdomyolysis due to immune hyperactivation and excessive cytokine release.13 The antibodies against SARS-CoV-2 spike glycoproteins cross-reacting with structurally similar host proteins (molecular mimicry) have been suggested as a possible cause for this acute autoimmune response. Other proposed mechanisms include cytokine-mediated autoinflammation, CD8 T-cell overactivation, formation of antigen–antibody complexes, and structural deformity of myocytes caused by intake of viral antigen.13 It has been suggested that the COVID-19 vaccination could also trigger such a response.14 However, the exact mechanism remains unclear. The clinical features suggestive of COVID-19-induced myositis include proximal muscle weakness, myalgia, features of muscle inflammation in MRI, dermatomyositis, and rhabdomyolysis evidenced by elevated CK and myoglobinuria.13
The Government of United Kingdom's “COVID-19 Vaccine AstraZeneca analysis print” reports one case of autoimmune myositis, 59 cases of myositis, six cases of polymyositis, and seven cases of dermatomyositis. These cases are reported during the period from 4th January 2021 to 13th July 2022. Theodorou et al.15 have reported a case like ours, with a 56-year-old woman presenting with myalgia and progressive muscle weakness that started 8 days after the second dose of COVID-19 vaccination. Her symptoms were limited to the left upper arm, where the vaccination was given. Myositis was diagnosed based on elevated CK and typical MRI findings. In our case, in addition to the edema at the injection site, edema was also observed in the ipsilateral calf muscle, which could not be explained otherwise. These features were suggestive of a generalized myositis. Maramattom et al.16 have recently reported three similar cases, all of which showed features of inflammatory myositis on MRI, while one patient showed features of vasculitis in addition to myositis on biopsy. Interestingly, the CK levels were normal in all the three cases. A comparison of these cases and few other published reports of ChAdOx1 nCoV-19-related myositis ranging from mild to fatal reactions is given in Table 1.
| Author | Clinicals | Biochemical parameters | Imaging | Management |
|---|---|---|---|---|
| Present report. | 53-year-old male, severe left upper arm pain, generalized weakness and myalgia 2 days after first dose. | Mildly elevated CK and ALT. | MRI: edema and perifascial fluid in left deltoid and left gastrocnemius muscles. | Rest, NSAIDs. |
| D.J. Theodorou et al. 2021, Greece. | 56-year-old female, severe left upper arm pain 8 days after second dose. | Elevated serum CK. | MRI: diffuse edema and intense focal enhancement of left deltoid. | Rest, cryotherapy, compression, and NSAIDs. |
| Maramattom et al. 2021, India. | 74-year-old male and 75-year-old female, fever, tachycardia, arthralgia, and myalgia 2 days after first dose. | Elevated AST and ALT, normal CK. Myositis panel negative. | 18 FDG-PET CT: FDG avid vessels in lower limbs (vasculitis). MRI: Patchy STIR hyperintensity involving bilateral lower limb muscles. | Oral prednisolone +/−mycophenolate mofetil. |
| 80-year-old female, fever, fatigue, and tachycardia 2 days after first dose. | Elevated LDH, AST and ALT, normal CK. Myositis panel negative. |
18 FDG-PET CT: Diffuse patchy minimally increased FDG avidity in skeletal muscles. MRI: Multiple patchy areas of STIR hyperintensity involving bilateral lower limb muscles. |
Oral prednisolone +/−Mycophenolate mofetil. | |
| M Capassoni et al. 2021, Italy. | 37-year-old female, skin rash, muscle weakness, and foot pain 4 days after vaccination. | Positive ANA, elevated aldolase. | Imaging not done. Histology: neutrophilic pustular dermatitis. EMG: Myositis in the tibialis anterior muscle. | Methylprednisone infusion and subsequent oral tapering. |
| S.-T. Huang, T.-J. Lee, K.-H. Chen et al. 2022, Taiwan. | 44-year-old male, generalized myalgia, progressive weakness, and brown urine 2 weeks after second dose. Bilateral forearm and gastrocnemius muscles tense and tender. | Markedly elevated CK and severe metabolic acidosis. Myositis panel positive. | CT: bilateral retroperitoneal fluid collection and swelling and edema of psoas muscles. | Emergent fasciotomy over four limbs, Methylprednisolone, Cyclophosphamide.Patient died of sepsis and multiorgan failure on day 17. |
A similar case of myositis with mildly elevated CK has been reported by Ramalingam et al17 following the second dose of mRNA vaccine. Laboratory tests revealed CK 236 U/L (reference range 35–232 U/L) and Aldolase 20.9 U/L (reference range 1.5–8.1 U/L). MRI showed diffuse cellulitis and myositis of the deltoid and supraspinatus muscles. The patient underwent local incision and drainage, along with antibiotic and steroid therapy. A summary of few other reported cases of myositis following other types of COVID-19 vaccinations is given in Table 2.
| Author | Vaccine type | Salient features |
|---|---|---|
| Present report. | ChAdOx 1 nCoV 19 (Serum Instituite of India) Recombinant, viral vector. | Severe injection site pain, generalized weakness, and myalgia. Mildly elevated CK and ALT. Features of inflammation at the site of injection as well as in the ipsilateral gastrocnemius muscle on imaging. |
| J H Kim et al. 2022, South Korea. | Pfizer-BioNTech (BNT162b2) mRNA vaccine. | Fever, skin rash, polymyalgia. Elevated CK, AST, ALT, LDH. Myoglobinuria. Generalized inflammatory myositis with signal changes in multiple groups of bilateral lower limb muscles and head and neck muscles on imaging. Rhabdomyolysis. |
| Tan et al. 2022, Malaysia. | CoronaVac COVID-19 Vaccine (Sinovac Biotech) Whole inactivated virus. | Bilateral proximal upper and lower limb weakness. Markedly elevated CK and Anti-SRP antibody. Features of myositis supported by histopathology and EMG (deltoid and iliopsoas muscles). |
| Ramalingam et al. 2021, USA. | mRNA COVID-19 vaccine | Redness and swelling at the injection site later spreading to whole arm. CK 236 U/L (reference range 35–232 U/L). MRI showed diffuse cellulitis and myositis of the deltoid and supraspinatus muscle. |
CK levels in myositis are variable. Though CK levels are raised in most cases of inflammatory myopathy, they may be minimally elevated or even normal in some cases, like inclusion myositis. Although injection site myositis has been reported following various vaccinations, it is usually associated with elevated CK. Our case showed features of myositis at the injection site and in other muscles following the COVID-19 vaccination, though CK was only mildly raised, with radiologic evidence of myositis and considerable patient symptoms and activity limitation. This merited medical attention since ChAdOx 1 nCoV 19 is a newly developed vaccine that is under Emergency Use Authorization.
Although muscle biopsy was not performed in our patient, the temporal link between the vaccination procedure and the patient's symptoms, imaging features, the reversible nature of symptoms, and lack of alternative cause suggests a diagnosis of COVID-19 vaccine-associated myositis as the cause. In addition, the other possible causes of muscle edema, like trauma, infection, neoplastic infiltration, and infarction (as in microvascular disease like diabetes mellitus), were not present in this case. Though the symptoms were mild, they incapacitated the patient for some time, resulting in a loss of motor function, which was relieved with rest and NSAIDs, not requiring treatment with steroids.
4 CONCLUSION
Most of the ChAdOx1 nCoV-19 vaccine-associated adverse events are mild and self-limiting. Prolongation or worsening of symptoms necessitate further evaluation. In our case, the myositis was mild, and the patient’s condition improved with rest and NSAIDs. Given the mild nature of the illness, many such cases may not even be reported. Evaluation of such patients with basic laboratory tests and imaging and follow-up will help in early diagnosis and management, preventing complications and reducing overall patient expense and better understanding of the unprecedented effects of this novel vaccine. Although such rare adverse events can be seen following the vaccination, it is not a deterrent factor for the ongoing vaccination programme.
AUTHOR CONTRIBUTIONS
Ponnu Bose: aquisition of data, interpreting and evaluating the MRI images, and drafting the original manuscript. Saibal Moitra: patient management and review and editing of the original draft. Usha Goenka, Sanjib Majumdar, and Srijita Ghosh Sen: interpretation and evaluation of the MRI images and editing and supervision of the original draft. Mahesh Kumar Goenka: patient management and review and editing of the original draft.
ACKNOWLEDGMENTS
A preprint of this case report is published online. It can be accessed here: https://www.authorea.com/doi/full/10.22541/au.164873236.66369928/v1
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Our institution does not require ethical approval for reporting individual cases or case series.
CONSENT
Written informed consent was obtained from the patient for his anonymized information to be published in this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study