Salmonella infantis ulcerative keratitis in a dog
Abstract
This manuscript describes a previously unreported clinical case of Salmonella enterica serovar infantis associated with ulcerative keratitis in a dog. Local immunosuppression of the corneal surface likely contributed to this opportunistic infection. Antimicrobial therapy with topical ofloxacin, tobramycin, and systemic enrofloxacin was successful in resolving the infection.
1 INTRODUCTION
Salmonella spp. are non-spore-forming, generally motile, gram-negative bacilli that can affect a wide variety of mammals, birds, and reptiles. The classification of salmonellae has undergone several changes, but currently there are only two species reported: Salmonella enterica and Salmonella bongori. Within the species S. enterica, there are over 2400 identified serotypes, identified by their flagellar and somatic antigens.1 While salmonellae are primarily intestinal pathogens that cause gastroenteritis, they can cause systemic disease, such as shock, cardiovascular collapse, central nervous system signs, septicemia, and death. Salmonellosis is a disease of major zoonotic importance, with infants, geriatrics, and immunocompromised individuals being at highest risk for severe disease. Poultry and their products (i.e., meat for consumption, eggs, etc.) are some of the highest sources of infection of both humans and animal species. The practice of feeding raw meat diets to dogs and cats can also increase the risk of infection and potential transmission to humans.1, 2
While salmonellosis can cause a wide variety of clinical signs in humans and veterinary species, Salmonella spp. are not commonly associated with ocular pathology. There is one reported case of Salmonella arizonae, primarily a pathogen of sheep, cultured from a horse with unilateral ulcerative keratitis. Salmonella was not a component of the normal bacterial flora of the horse's ocular surface and the horse did have reported direct contact with sheep.3 There are reported studies of Salmonella spp. (specifically S. typhimurium and S. weltevreden) capable of producing keratoconjunctivitis in guinea pigs.4, 5 In dogs with bacterial keratitis, common isolates are Staphylococcus intermedius, β-hemolytic Streptococcus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae.6-8 To the authors' knowledge, Salmonella has not been cultured from a dog with ulcerative keratitis. In this case report, Salmonella infantis was isolated from an infected corneal ulcer of a dog. The clinical findings, results, and treatments involved are described.
2 CASE REPORT
An 11-year-old neutered male mixed breed dog was presented to the ophthalmology service at Oklahoma State University Veterinary Teaching Hospital for evaluation of blepharospasm of the left eye (OS) identified by the owner for 1 week and an opacity OS noted for 3 days. The dog was previously evaluated for hypermature cataracts in both eyes (OU) secondary to diabetes mellitus. Bilateral phacoemulsification with intraocular lens implantation was performed 5 months prior to evaluation for blepharospasm and corneal opacity OS. The dog was being treated with prednisolone acetate 1% ophthalmic suspension one drop OU q48h (Alcon Laboratories, Sandoz Inc.), ketorolac 0.5% ophthalmic solution one drop OU q12h (Sun Pharmaceutical Ind.), 4.5 units Vetsulin (Merck Animal Health) administered subcutaneously twice daily, and enalapril (dose unknown).
Complete ophthalmic examination including slit-lamp biomicroscopy (SL-17 Portable Slit Lamp), tonometry (Tonovet®, Jorgensen Labs), fluorescein stain (Bio-Glo™ sterile fluorescein strips, HUB pharmaceuticals), and indirect ophthalmoscopy (Keeler binocular indirect ophthalmoscope, Keeler Ophthalmic Instruments) was performed on both eyes. Schirmer tear test-1 (STT-1) (Schirmer tear test strips®, Merck & Co. Inc.) was not performed, but at an evaluation 1 month prior, these values were normal OU (20 mm/min OD, 15 mm/min OS). Menace response and palpebral reflex were positive OU. Pupillary light reflexes (PLR) were positive OD, and negative OS due to an efferent PLR deficit associated with posterior synechia. Intraocular pressure was 12 mmHg OD and 8 mmHg OS. Moderate blepharospasm and conjunctival hyperemia were noted OS, along with robust corneal neovascularization extending from the dorsal limbus leading to a corneal facet with approximately 40% stromal loss. Immediately ventral to the facet was a dense, creamy white, elevated corneal opacity (plaque) that was fluorescein stain positive. Previously diagnosed lipid keratopathy was noted axially within the superficial stroma, as well multifocal posterior synechiae diagnosed following phacoemulsification. The remainder of the ophthalmic examination was unremarkable. Clinical abnormalities of the right eye (OD) were limited to a small, pigmented eyelid margin mass, mild lipid keratopathy, and pseudophakia. A cytology sample was obtained from the corneal ulcer, and microscopic examination revealed abundant rod-shaped bacteria and rare polymorphonuclear leukocytes (PMNs). An aerobic culture and susceptibility profile was submitted on a corneal swab of the ulcer. The owner elected to pursue aggressive outpatient medical management. Due to the evidence of adjacent stromal loss and abundant bacterial organisms demonstrated on corneal cytologic evaluation, intensive topical antimicrobial therapy was initiated (dosing q1-2h).9 Therapy included ofloxacin 0.3% ophthalmic solution (Akorn Inc.) one drop OS q1-2h, tobramycin 0.3% ophthalmic solution (Akorn Inc.) one drop OS q1-2h, serum (equine) one drop OS q1-2h, enrofloxacin (Baytril®, Bayer HealthCare LLC) 68 mg PO q24h, meloxicam (Metacam®) 0.6 mg PO q24h, and continued ketorolac OD only (one drop OD q12h) as previously prescribed.
Three days later, the left eye showed moderate hyperemia and blepharospasm. The dense cellular infiltrate associated with the corneal ulcer was still present, and no additional progress toward epithelialization of the ulcer was noted. Superficial stromal vasculature had progressed beyond the dorsal aspect of the corneal facet toward the dense cellular infiltrate (Figure 1). No changes were made to the therapeutic plan at this time. Another recheck examination was performed 6 days after the ulcer was diagnosed. Examination OS showed moderate blepharospasm, moderate conjunctival hyperemia, improved corneal edema, cellularity still present at the ventral aspect of the facet, and no remaining elevated plaque present. Corneal neovascularization had reached the corneal cellular infiltrate, and epithelialization of the corneal ulcer had progressed. A repeat in-hospital cytological examination showed increased neutrophils and no organisms. At this time, preliminary culture results showed Salmonella spp. Based on this finding, a conjunctival swab OD and a fecal sample were obtained and submitted to the Oklahoma Animal Disease Diagnostic Laboratory for Salmonella culture. The samples were enriched at 42°C in Tetrathionate broth. The inoculated broth was then plated on XLT and Brilliant Green plates, which are the selective and indicative media for Salmonella. Both the fecal and OD conjunctival swab samples were reported negative for Salmonella spp. after 3 days of no bacterial growth. The culture and susceptibility panel from the corneal ulcer OS showed that the Salmonella spp. was sensitive to fluoroquinolones and aminoglycosides. PCR speciated the cultured organism as Salmonella enterica Serovar Infantis (Salmonella infantis). Frequency of ofloxacin and tobramycin ophthalmic solution was reduced to one drop OS q2h; all other medications were maintained at the same dose and frequency.
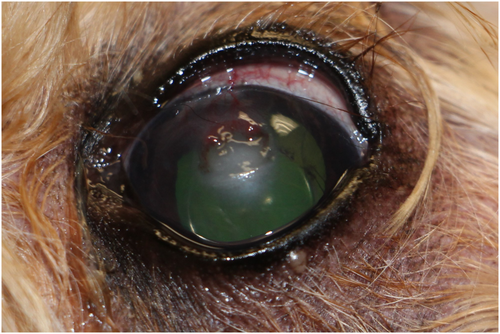
Ten days after the infected corneal ulcer diagnosis, the left eye showed mild blepharospasm and conjunctival hyperemia. The previous site of ulceration was fluorescein negative. There was persistent neovascularization and improved corneal edema and cellular infiltrate. At this time, tobramycin ophthalmic solution and serum were discontinued, ofloxacin dosing was decreased to one drop OS eight times per day, meloxicam was discontinued, and enrofloxacin was continued at 68 mg PO q24h for a total of 2 weeks. Subsequent recheck examinations showed improved ocular comfort, corneal fibrosis, and remodeling of the corneal facet (Figures 2 and 3). Ofloxacin ophthalmic solution was gradually tapered and discontinued.
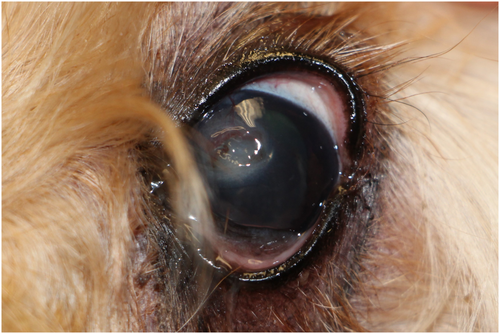
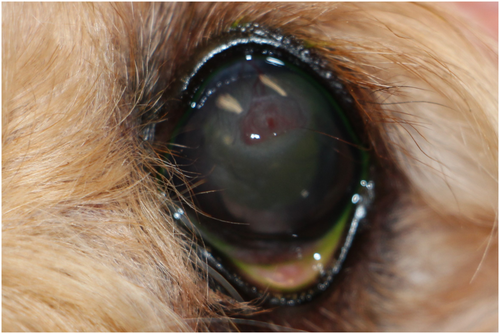
Two months after the ulcer was diagnosed, a Schirmer tear test-1 (STT-1) revealed decreased tear production (11 mm/min) OD and normal tear production (22 mm/min) OS. The dog was prescribed cyclosporine 1% ophthalmic solution in MCT oil (Stokes Compounding Pharmacy) one drop OD q12h. Four and a half months after the ulcer was noted, decreased tear production (13 mm/min) was diagnosed OS and the dog was placed on cyclosporine in both eyes.
Interestingly, 5 months after the initial corneal ulcer was noted in the left eye, the owner reported cloudiness in the right eye. Ophthalmic examination revealed severe blepharospasm, severe conjunctival hyperemia, a densely cellular corneal plaque associated with the ventral aspect of a mid-stromal corneal ulcer, robust vessels extending dorsally, miotic pupil, and 2+ aqueous flare OD. A culture and sensitivity were submitted on a corneal swab, conjunctival swab, and a fecal sample for suspected Salmonella infection as previously identified in the prior corneal ulcer. The conjunctiva and fecal samples were negative for Salmonella and the ulcer sample grew K. pneumoniae. The ulcer healed uneventfully with medical therapy.
3 DISCUSSION
Ulcerative keratitis complicated by infection of Salmonella spp. is an unreported finding in dogs and there are very minimal reports of ocular infections associated with Salmonella spp. in other species.3-5 A thorough history was obtained from the owner of the dog described in this report, and there were no specific risk factors for fecal contamination of the dog's ocular surface. However, in dogs, there are opportunities for fecal contamination of the ocular surface associated with normal behaviors and the animal's environment (for example, rubbing its face in grass where fecal contaminants are present). Because of concerns for both systemic disease development (Salmonellosis) and zoonotic transmission, after the preliminary culture results showed Salmonella, additional samples were taken from the conjunctiva of the other eye and feces in an attempt to find other sources of infection. No other submitted samples were positive for Salmonella. It should be noted that the patient was treated with oral enrofloxacin prior to conjunctival and fecal sample collection.
Salmonella spp. can contaminate produce and food-producing animals. Poultry are considered one of the most important sources of infection. Improperly packaging, storing, handling, cooking, or cleaning utensils used with raw meat products puts humans at risk of developing food-borne illness. S. infantis is one of the most common isolates responsible for illness associated with human salmonellosis in both the United States and Europe.10 There is growing concern of multidrug resistance of this serovar. When evaluating a global collection of S. infantis, a cluster with strains predominantly from human and avian sources contained a pESI-like plasmid containing multiple resistance genes.11 In recent years, homemade diets, particularly raw diets, have become increasingly popular with pet owners. This is concerning because both humans and animals can be exposed to food-borne illness from raw meat. Not only are homemade raw diets at risk, but commercially made raw diets can pose the same threat. A Canadian study found multiple Salmonella serotypes in both Canadian- and American-made raw dog food brands, with chicken meat showing a higher prevalence.12 There are also reports of Salmonella in commercially available pet chews.13 The dog described in this case report was not on a raw diet and was not exposed to poultry or food-animal species.
In humans, the most common isolates of bacterial keratitis include S. aureus, Streptococcus pneumoniae, and gram-negative coliform bacteria (such as Klebsiella).14 Endogenous endophthalmitis is a rarely reported complication of Salmonella infections in humans. Salmonella-associated endophthalmitis is associated with a poor outcome, with all reported cases progressing to complete vision loss, and many cases requiring enucleation or evisceration.15, 16 In human medicine, studies have shown that the repeated use of topical antibiotics significantly alters the composition of the normal ocular flora and potentially allows for the colonization of other opportunistic and more virulent pathogens.17 Following phacoemulsification, the dog was treated with topical ofloxacin due to its broad spectrum coverage and its ability to penetrate an intact corneal epithelial layer.18 Topical fluoroquinolones are commonly used in human ophthalmology for post-operative care following cataract surgery. A study in humans evaluating ocular flora and antimicrobial resistance pattern in patients undergoing cataract surgery found that coagulase-negative staphylococci were the most commonly identified organisms. The overall susceptibility for these organisms was highest for gentamicin (94%), and 64.5% were sensitive to ciprofloxacin.19 A similar study in canine cataract surgery patients identified an increased proportion of organisms that developed resistance to ofloxacin after 3 weeks of topical ofloxacin administration every 6 h compared with the proportion prior to receiving any ofloxacin. The most frequently identified organism in this study was Staphylococcus pseudintermedius, followed by coagulase-negative Staphylococcus spp.20 The dog described in this report was treated with ofloxacin for 2 weeks post-cataract surgery, and was no longer receiving this antibiotic at the time ulcerative keratitis was diagnosed. It is unknown how long alteration of the normal ocular flora persists in patients following antimicrobial therapy associated with cataract surgery.
Additionally, while the initial cause of the first ulcer is not known, the dog was later diagnosed with keratoconjunctivitis sicca (KCS). Tears are required to maintain normal health and function of the cornea and contain components to provide antibacterial defense.21 Decreased tear production can lead to corneal ulceration, impaired reepithelialization, and increased number of conjunctival bacteria.22, 23 Both cyclosporine and tacrolimus have been demonstrated to be efficacious in the treatment in the canine KCS. The dog described in this case report responded well to cyclosporine and demonstrated improved tear production at follow-up evaluations.24
Diabetic dogs are at increased risk of inadequate or tear production due to decreased corneal sensitivity and are almost twice as likely to develop KCS following cataract surgery as compared to non-diabetic patients.25, 26 The dog described in this study weighed approximately 6 kg, and it has been documented that small breed dogs are predisposed to post-operative KCS development.26 Furthermore, dogs with diabetes mellitus have been shown to be at increased risk of infections, such as urinary tract infections and bacterial or yeast dermatitis. One possible mechanism proposed for an increased risk of infection in diabetic dogs is decreased neutrophil adherence, although the clinical significance of this finding has not been determined.27 Finally, it should also be noted that the dog was being treated with a topical corticosteroid, prednisolone acetate, at the time infectious ulcerative keratitis was diagnosed. Topical corticosteroids have been shown to decrease corneal epithelial cell migration and wound healing,28 and suppression of the normal corneal inflammatory response may have contributed to the development of bacterial keratitis.
In conclusion, our findings suggest that Salmonella spp. should be considered when treating complicated ulcerative keratitis in dogs, and appropriate precautions should be observed to minimize the risk of zoonosis associated with these bacteria.
AUTHOR CONTRIBUTIONS
Brayden L. Routh and Emily S. McCool contributed to management and evaluation of case material and drafting, revision, and final approval of the manuscript.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
ETHICAL APPROVAL
This case report and the patient care described therein conform to institutional ethical guidelines. Permission was granted by the owner of the dog described for publication of this case report.
DISCLOSURES
There are no disclosures to make and there was no outside support provided for this report.
CONSENT
Informed consent for publication was obtained from the owner of the dog described in this report.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study