NTRK3 mutation in secretory carcinoma of the parotid gland: A Case report
Institution: Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA
Abstract
Mammary analog secretory carcinoma (MASC) is a newly described carcinoma with a molecular hallmark of ETV6-NTRK3 fusion that promotes oncogenesis. While MASC histopathology was well-studied in the literature, clinical behavior remains unstudied. We present a 22-year-old man with painless parotid mass, which was diagnosed as salivary gland cancer, MASC subtype.
1 INTRODUCTION
Skalova et al. first described secretory carcinoma of the salivary gland in 2010, as a mammary analog secretory carcinoma (MASC), based on the morphological and immunohistochemical features that resemble secretary carcinoma of the breast.1
The unique features of MASC include the absence of zymogen granules, strong staining for mammaglobin, and the presence of abundant extracellular colloid-like material, which are common features of secretary breast carcinoma (SBC). In addition to sharing these morphological features with the SBC, both carcinomas commonly express ETV6-NTRK3 gene fusion,2, 3 which is caused by translocation breakpoints of the t(12;15)(p13;q25). The fusion results in a constitutively active chimeric tyrosine kinase and probable activation of the Ras-MAP kinase mitogenic pathway and the phosphatidylinositol-3-kinase-AKT pathway, which is believed to drive the oncogenesis process. We find a similar gene alteration in other tumors such as infantile fibrosarcoma, myelogenous leukemia, and congenital mesoblastic nephroma.4, 5
MASC commonly arises in the major salivary glands, among which the parotid gland is the most affected. The clinical behavior of MASC in the medical literature remains understudied. In this case report, we present a case of a MASC and we discuss the common clinical features of MASC found in the literature.
2 CASE PRESENTATION
A 22-year-old man presents with a one-year history of right-sided parotid mass and neck swelling. Initially, the parotid gland was painless with minimal discomfort, so he did not seek medical attention. However, when the neck swelling started to increase, he sought medical attention. The patient's family and social history was unremarkable. He had no prior radiation exposure. On examination, the parotid mass was visually noticeable, with palpable, non-tender, and non-fixable swelling located at the right mandible angle.
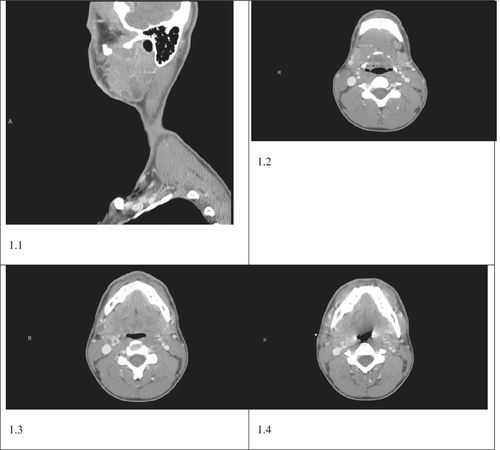
There was no other significant lymphadenopathy. The rest of the physical examination was unremarkable. Initial laboratory evaluation including complete metabolic panel and complete blood count was within normal limits. A neck CT scan was obtained and revealed 2.9 × 3.1 cm superficial hypo-enhancing mass involving the tail of the right parotid gland (see Figure 1.1) accompanied by ipsilateral cervical lymphadenopathy involving Ib and IIa lymphatic stations (see Figure 1.2–1.4). CT findings were consistent with primary parotid neoplasm with subsequent nodal metastasis. Consequently, we obtained a fine needle aspiration biopsy, which was suspicious for malignancy. He later underwent superficial parotidectomy along with modified radical lymph node dissection.
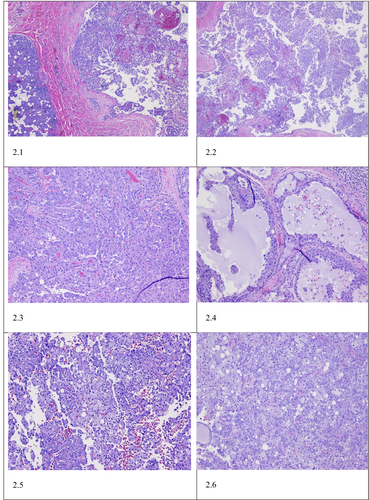
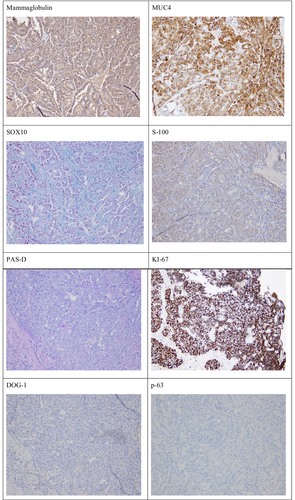
Gross examination of the parotidectomy specimen showed a single, well-circumscribed, soft to friable mass within the parotid gland measuring 2.8 cm in maximum dimension. Microscopic analysis of the specimen revealed a non-encapsulated lesion with solid, microcytic, including papillary cystic growth of neoplastic cells. The tumor cells showed clear eosinophilic cytoplasm with multiple foamy to bubbly secretions with the cytoplasm. Colloid-type secretion was present within the cystic spaces and glandular lumen (see Figure 2). Immunohistochemical staining of the tumor cells showed tumor cells to stain-positive for mammaglobin, MUC4, SOX10, and S-100. The tumor cells showed weak staining with GATA-3, and no staining for DOG-1, p63. Pas-D stained the glandular secretions (see Figure 3). A diagnosis of secretory carcinoma, T2PN3BM0, stage II was rendered.
As part of the tumor evaluation, comprehensive molecular testing was performed on the tumor tissue. Immunohistochemical (IHC) staining was positive for MLH1, MSH2, MSH6, and PMS2, suggesting intact mismatch repair (MMR) machinery. Furthermore, IHC staining showed negative AR, ERBB2, and PDL1 staining. DNA mutation analysis showed that the tumor is MSI stable, has low TMB, and somatic mutations in EML4 (c.1252C > T, H418Y), EPHB4 (c.2324C > T, T775M), ERCC5 (c.2911A > G, T971A), GLI2 (c.649C > G, R217G), HOOK3 (c.168 T > G, N56K), MLLT3 (c.1331G > A, R444K), PCM1 (c.6033_6034delGA, E2011fs), SMARC4 (c.220A > C, K74Q), and ZFHX3 (c.2330_2347del18, V777_A782del). mRNA fusion analysis identified a pathogenic ETV6:NTRK3 fusion that is typically identified in MASC tumors, with exon 5 of ETV6 joined in-frame to exon 15 of NTRK3. Germline testing showed no clinically significant variants detected.
The patient then proceeded to receive post-surgical adjuvant chemo-radiotherapy (CRT) with weekly carboplatin and paclitaxel. The patient completed the prescribed CRT. A follow-up imaging study, after 12 weeks of completing CRT, revealed no evidence of disease.
3 DISCUSSION
The neurotrophic tyrosine receptor kinase genes 1, 2, and 3 (NTRK1, NTRK2, and NTRK3) encodes the tropomyosin-related kinases. These receptors play a crucial role in the function of the peripheral and central nervous systems, both physiologically and developmentally.6, 7 When NTRKs are activated, they promote proliferation, differentiation, and survival of cells. The fusion of the transcriptional regulator gene ETV6 and the membrane receptor kinase NTRK3 results in a chimeric tyrosine kinase that continuously promotes cell proliferation and increases its survival playing a fundamental role as an oncogene.8
Approximately 90 cases of MASC have been reported. Among these cases, there is a notable male predominance.9, 10 The most commonly affected gland is the parotid, accounting for two-thirds of the reported cases.11 It usually presents as an enlarging non-tender mass that is detected incidentally on physical examination.12 MASC normally does not have aggressive behavior, so it is considered a low-grade carcinoma with a favorable prognosis, with moderate risk for local recurrence (15%), lymph node metastases (20%), and a low risk for distant metastases (5%).10
MASC is commonly diagnosed by confirming the t(12;15) (p13;q25), this translocation, as stated above, results in the ETV6-NTRK3 gene fusion.13 Another approach is to confirm the presence of positive immunohistochemical studies for STAT5, mammaglobin, and S-100 protein.14 It should be noted that a negative test for ETV6-NTRK3 gene fusion does not rule out the diagnosis of MASC. One of the main differential diagnoses to MASC is low-grade salivary duct carcinomas or acinic cell carcinomas (ACC). The characteristics of ACC are the presence of large, serous, acinar cells with cytoplasmic PAS-positive, zymogen-like granules, these are usually absent in MASC.15 Histologically, MASC is characterized by the proliferation of uniform eosinophilic cells with a vacuolated cytoplasm, growing within a microcystic, macrocystic, and papillary architecture.16 The growth rate between MASC and ACC is similar; however, MASC is more likely to metastasize to the regional lymph nodes. Hence, MASC is more aggressive tumor. The disease-free period for MASC ranges from 71 to 115 months, which is shorter than ACC, which is 92–148 months.17
The treatment approach for MASC has not been well defined or standardized. The reason for this is that most studies in the literature have been retrospective.16 Various treatment approaches to manage MASC are usually dependent on the tumor grade and range of surgical resection, non-radical vs radical resection, radiotherapy to neck dissection or chemotherapy. The treatment of choice in high-grade transformation MASC has been radical surgery with neck dissection in addition to adjuvant radiotherapy.16 In the future, using ETV6-NTRK3 inhibitors might become a therapeutic target. The anti-tumor activity of tropomyosin receptor kinase (TRK) inhibitors has shown a robust response for patients with NTRK-rearranged tumors in clinical trials. Specifically, larotrectinib and entrectinib are very potent and promising TRK inhibitors. Targeting TKR proteins is a promising potential treatment option specifically for patients with unresectable MASC. The question is what is the value of TRK inhibitors in the adjuvant setting for a patient with no evidence of disease after surgery and post-surgical radiotherapy, as in our patient’s case.
AUTHOR CONTRIBUTIONS
Fahad AlKaabba wrote the manuscript and coordinated to prepare the study. Abdurahman Alloghbi reviewed the manuscript, prepared the slides, and coordinated to prepare the study. Yazeed Deajim wrote the manuscript. Mohamad W Sukkari and Ammar Sukari reviewed the manuscript. Mir Yousufuddin Ali Khan reviewed the molecular studies and prepared the slides.
ACKNOWLEDGMENT
None.
FUNDING INFORMATION
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
All data underlying the results are available as part of the article, and no additional source data are required.