First report of novel mutation (c.790del) on SQSTM1 gene on a family with childhood onset of progressive cerebellar ataxia with vertical gaze palsy
Abstract
SQSTM1 gene encodes a protein called p62 that acts as an autophagy receptor in the degradation of protein molecules. A homozygous deletion variant that changes the frame shift in the SQSTM1 gene named c.790 Del A .T was detected in case childhood onset and progressive neurodegeneration with ataxia, and gaze palsy.
1 INTRODUCTION
Located on long arm of chromosome 5 (5q35.3), the SQSTM1 gene encodes a protein called p62 that was first identified by Jaekyoon Shin and his colleagues.1 P62, also known as sequestosome-1, is a scaffold protein, playing a pivotal role in modulating enzyme function through several domain interactions. P62 acts as an autophagy receptor in the degradation of protein molecules. In the past decade, studies have shown that mutations on SQSTM1 gene are associated with several diseases including Paget's disease of bone (PDB), liver cancer, breast cancer, obesity, diabetes, and neurodegenerative diseases (including Ataxia, Dystonia, And Gaze Palsy, Childhood-Onset).2
With the advent of next-generation sequencing (NGS), a larger number of the new mutations causing various syndromes were identified. In the presented case, we describe a patient from north of Iran who presented with progressive cerebellar ataxia and vertical gaze palsy due to homozygous SQSTM1 mutation detected by WES (Whole Exome Sequencing).
2 CASE PRESENTATION
A 53-year-old female patient with childhood onset and progressive neurodegeneration with ataxia, dystonia, and gaze palsy was referred for genetic counseling and subsequent DNA analysis. The patients had severe scoliosis, short neck, ataxia, oculomotor apraxia, dystonia, dysmetria, dysarthria, cognitive abnormality, Pes Cavus, and Dysdiadochokinesia.
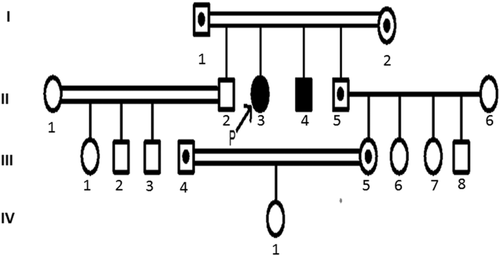
She was born of a consanguineous marriage, and family history showed that she had a brother with similar clinical manifestations (Figure 1).
A written informed consent was obtained and in order to explore the probable disease-causing variants, Genomic DNA was isolated from the whole blood sample, and whole-exome sequencing (WES) was conducted by Illumina platform. A homozygous deletion variant that changes the frame shift in the SQSTM1 gene named c.790del was detected. Analysis of the identified variant using Mutation Taster tools predicted the variant as a disease-causing frame-shift mutation.
In order to verify the identified variant and explore the mutation among other members of the family, polymerase chain reaction (PCR) amplification and targeted sequencing of the identified variant were applied. Accordingly, the DNA sequence of SQSTM1 gene was obtained from the NCBI database, and locus-specific primers (5-CTGAATTGGAGAAAGAGAAAGG-3 and 5-AAGGCGATCTTCCTCATCTG-3) were designed using the Oligo7 software. Then, the samples were sequenced by Sanger sequencing method via Applied Biosystems 3130xl Genetic Analyzers. Finally, the obtained sequences were assessed by Codon code software. We also detected the mutation in heterozygote state in two nieces of the affected case who got married to each other. Although the carrier couples have a normal girl, the prenatal diagnosis for further pregnancies is recommended (Figure 2).

3 DISCUSSION
Neurodegenerative diseases that result from the progressive loss of function and subsequent death of neurons in the central and peripheral nervous systems cause major threats to human health including severe disability, or even early death.3 The identification of related genetic variants in both common and rare cases provides crucial new insights into pathophysiology and basic cellular processes of the disease. Almost in all of the neurodegenerative diseases, ubiquitin-enriched misfolded protein inclusions are main cellular characteristics.4 P62 is an adaptor protein for ubiquitinated substrates selected for Macroautophagy.5 The absence of p62 was observed in patients with childhood-onset neurodegenerative disease6 and SQSTM1 knockdown in zebrafish model leads to the neurodegenerative disorders ALS/FTLD in which abnormal motor behavior and shortening of motor neurons were obvious.7, 8
In 2016, Haack et al. for the first time described childhood and adolescence onset of neurodegenerative syndrome, demonstrating mainly with gait abnormalities and ataxia in four different families. Exome sequencing in nine subjects showed three different biallelic loss of function variants in SQSTM1 gene6: The c.286C>T and c.311_312del variants that affect all three predicted SQSTM1 isoforms and c.2T>A variant that only affects the start codon of one isoform. Muto et al. in 2018 detected 11 affected individuals from three unrelated consanguineous families two of which were from central Iran where the c.934_936delinsTGA and c.875_876insT mutations were identified in the affected cases and another family from Italy with c.301+2T>A splice site mutation (Table 1).9 Cerebellar ataxia, dysarthria, cognitive impairment, and gaze palsy were observed among Iranian patients while in the presented case in addition to the mentioned clinical manifestations dystonia and dyskinesia were also detected. Moreover, Iridoplegia, dysautonomic features such as orthostatic hypotension and sudomotor dysfunction, along with other non-motor symptoms was also reported in patients from Mexico and Jourdan that was not observed in the presented case.
| Pathogenic variant | Origin | Confirmed cases | Age on onset | Location on the gene |
|---|---|---|---|---|
| c.790del | North of Iran (presented case) | 1 | Early childhood | Exon 7 |
| c.311_312del | United Arab Emirates | 3 | 10, 10, 10 | Exon 3 |
| c.286C>T | Finland, Kurdish Origin | 3 | 7, 8, 8 | Exon 2 |
| c.2T>A | Germany | 3 | 10, 12, 14 | Exon 1 |
| c.934_936delinsTGA | Central Iran | 2 | 10, 12 | Exon 6 |
| c.875_876insT | Central Iran | 7 | 10 to 11 | Exon 6 |
| c.301+2T>A | Italy | 2 | 6 and 12 | Intron 2 |
| c.712_713insTCCTCCGAGTGTGAATTTCCTGA | India | 1 | 11 | Exon 5 |
Some variants of SQSTM1 gene are common to both PDB and ALS/FTD, others have been suggested to be ALS/FTD specific,10 and in rare cases like the presented case, the mutation was reported linked to childhood-onset progressive cerebellar ataxia with vertical gaze palsy. The confusing variety of clinical symptoms and inheritance patterns associated with SQSTM1 variants indicates complex genotype/phenotype relationships and introducing the new variants can leads to the better understanding of the gene function.
AUTHOR CONTRIBUTIONS
Hossein Jalali performed the test and analyzed the results, and involved in writing of the article. Atefeh Khoshaeen visited the case as a physician. Mohammad Reza Mahdavi involved in writing and editing of the article. Mahan Mahdavi performed the tests.
ACKNOWLEDGMENT
The authors would like to thank the staffs of Fajr Medical genetics and Pathobiology Laboratory.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ETHICAL APPROVAL
Written informed consent was obtained from the patient's brother.
CONSENT
Written informed consent was obtained from the patient's brother to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
All data underlying this article are incorporated into the article.