Giant cell myocarditis following COVID-19 successfully treated by immunosuppressive therapy
Abstract
It has been shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), by coronavirus disease 2019 (COVID-19), can lead to multi-organ impairment including cardiac involvement and immunological problems. Acute myocarditis is one of serious and fatal complications of COVID-19. In this case report, we present a 46-year-old lady with a history of lichen planus dermatitis who has developed a rapidly progressive heart failure after an episode of COVID-19. The pathologic examination of her endomyocardial biopsy specimens was compatible with GCM, and she was successfully treated with a combined immunosuppressive therapy regimen.
1 INTRODUCTION
Giant cell myocarditis (GCM) is a rare and rapidly progressing form of myocarditis of autoimmune origin, histologically characterized by the presence of giant multinucleated cells along with extensive inflammatory infiltrates and is often associated with autoimmune disorder.1-4
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused by coronavirus disease 2019 (COVID-19), a clinical syndrome with hallmark of respiratory system involvement and different patterns of lung injury.5 In addition to respiratory system, COVID-19 can lead to the other organs impairment such as cardiovascular injury. Cardiac injury occurs in about 30% of these patients and includes myocardial infarction, arrhythmia, myocarditis, acute heart failure, and sudden cardiac death. COVID-19 has been considered as an established etiology of acute myocarditis.5-9 However, there is only one case report other than the present case regarding the development of GCM following COVID-19.10 Here, we are going to report a case of GCM following COVID-19 infection in a lady with a history of lichen planus dermatitis and hypothyroidism.
2 CASE PRESENTATION
A 46-year-old female patient referred to heart failure clinic for further evaluations of her new onset heart failure. In her first visit on January 2021, she complained of a dyspnea of exertion, New York Heart Association functional class of II (NYHA-FC) and fatigue.
Her past medical history was unremarkable in terms of cardiovascular problems before her recent episode of acute heart failure. She had a history of a controlled lichen planus dermatitis (LP) treated by immunosuppressive therapy several years ago. She also had a history of antithyroid peroxidase antibody (TPO) positive hypothyroidism that was managed with levothyroxine.
A month before the first visit in our clinic (on December 2020), she had developed symptoms of COVID-19 such as fever, sore throat, and malaise. The real-time polymerase chain reaction (RT-PCR) test for the novel coronavirus infection was positive but the clinical course of her COVID-19 infection was uneventful without any decrease in arterial O2 saturation or pulmonary involvement and the disease was subsided using the recommended medications for mild COVID-19.
About three weeks later (a week after the remission of the COVID-19 infection), she was admitted due to a severe chest discomfort and shortness of breath. Her electrocardiogram (ECG) was highly suggestive of acute anterior myocardial infarction (ST segment elevation in V2-V6 leads), according to her hospital discharge note. Considering the ECG changes and an elevated cardiac Troponin-I level, she underwent emergent coronary angiography, which revealed normal epicardial coronary arteries. Her echocardiogram reported a normal left ventricular (LV) size, with apical hypokinesia and an ejection fraction (EF) of 35–40%, normal right ventricular (RV) size and function, a mild mitral valve regurgitation (MR), a mild tricuspid valve regurgitation (TR), no pulmonary hypertension, and no pericardial effusion.
With an impression of acute myocarditis, she underwent a cardiac magnetic resonance imaging (CMR), and based on the results (Figure 1; Video S1), she was referred to our heart failure clinic for further evaluations.
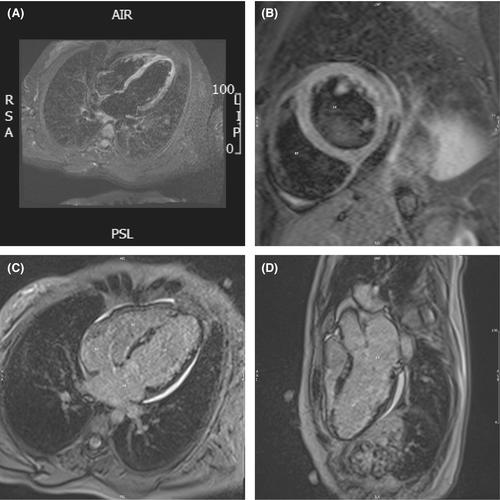
2.1 CMR report
The CMR report revealed mildly enlarged LV size without LV hypertrophy with moderately reduced LV systolic function LVEF = 35% and thin and aneurysmal LV apex –Normal RV size with mildly reduced RV function (RVEF = 42%).
LGE findings: In all inferolateral and septal segments, there was widespread patchy subepicardial to mid wall fibrosis, as well as subendocardial fibrosis in the left ventricular apex.
Recent myocarditis possibly due to viral infection, as well as an autoimmune or sarcoidosis disease, should be evaluated, according to CMR tissue characterization criteria.
On the first visit in our clinic on January 2021, she complained of a dyspnea on exertion, NYHA-FC II, and a mild fatigue. Her vital signs were stable with a blood pressure (BP) of 115/75 mmHg a heart rate (HR) of 90 beats per minute (bpm), she did not have fever, and systemic oxygen saturation was 96%.
Her physical examination was unremarkable except for bi-basilar fine crackles. She did not have any skin lesions in terms of her LP. Her new echocardiogram revealed no new changes compared to her last examination that was mentioned earlier. On laboratory tests, there was no leukocytosis, hemoglobin was 12.1 g/dl, the renal and liver function tests were within normal limits, and N terminal –pro natriuretic peptide (NT-pro BNP) was 885 pg/ml. The thyroid function test was within normal limits with a higher than normal anti-TPO.
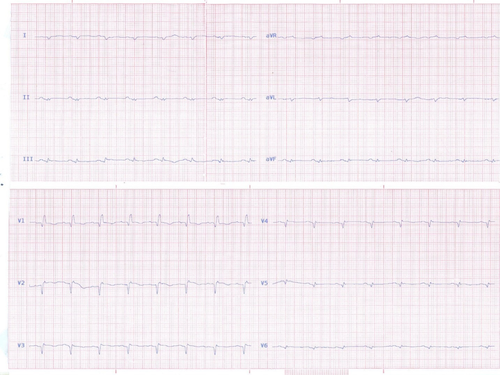
The standard anti-failure therapies were already started, and the heart failure (HF) guideline-directed medical therapies with lisinopril, bisoprolol, eplerenone, furosemide as well as levothyroxine were continued for her. A few days later, her HF symptoms were aggravated and she developed progressive dyspnea, orthopnea, paroxysmal nocturnal dyspnea, abdominal pain, and nausea. She was admitted with the abovementioned symptoms and rapidly progressed to a pre-shock state. She had a BP of 85/60, an HR of 120 bpm, a distended jugular vein, bi basilar lung crackles, hepatomegaly, mild ascites, and 2+ pretibial pitting edema. The creatinine was slightly increased (1.7–1.8 mg/dl). The alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin levels were 46 U/L, 40 U/L, and 1.5 mg/dl, respectively, NT-Pro BNP was more than 18,000 pg/ml, and cardiac troponin –I (CTnI) was elevated. Her ECG showed a sinus tachycardia with low voltage QRSs and Q wave in leads of I, III, aVF, V1-V6, ST segment elevations, and T wave inversions/flattening in V1-V6 (Figure 2).
New echocardiography showed mild LV enlargement with severe systolic dysfunction, LVEF 15%–20%, global hypokinesia, and apical akinesia mild RV enlargement with severe systolic dysfunction [Tricuspid annular plane systolic excursion(TAPSE) = 10 mm, RV peak systolic myocardial velocity by tissue Doppler (RV Sm) =6 cm/s], moderate to severe mitral (MR) and tricuspid regurgitation(TR), a tricuspid regurgitation gradient (TRG) of 25 mmHg and a plethoric inferior vena cava (IVC) with an IVC size of 23 millimeter.
Considering her clinical condition, we had to start intravenous (IV) inotrope and diuretic. After starting intravenous (IV) inotrope (Milrinone) and furosemide infusion, she was scheduled for emergent endomyocardial biopsy (EMBX) due to deterioration in hemodynamic and clinical course. The right heart catheterization data at the time of EMBX have been shown in Table 1.
| Catheterization data | Value |
|---|---|
| Cardiac output by Fick method | 2.4 Liters/minute |
| Cardiac index by Fick method | 1.4 Liters/kilogram of body weight/minute |
| Mean central venous pressure | 16 mmHg |
| Mean Right ventricular pressure | 32/10–20 mmHg |
| Pulmonary arterial pressure | 32/16 mmHg |
| Pulmonary capillary wedge pressure | 17 mmHg |
| Systemic arterial pressure | 90/60 mmHg |
| Mixed venous oxygen saturation | 48% |
| Systemic arterial saturation | 98% |
Five pieces of myocardial tissue from right ventricular side of interventricular septum were obtained and sent for both pathological examination and tissue polymerase chain reaction (PCR) for all cardiotropic viruses including influenza type A and B, adenovirus, enterovirus, parvo virus B-19, cytomegalovirus, human herpesvirus 6 (HHV-6), and the novel coronal virus.
Tissue PCR for all cardiotropic pathogens and the novel corona were negative. Autoimmune and connective tissue disease serology was unremarkable.
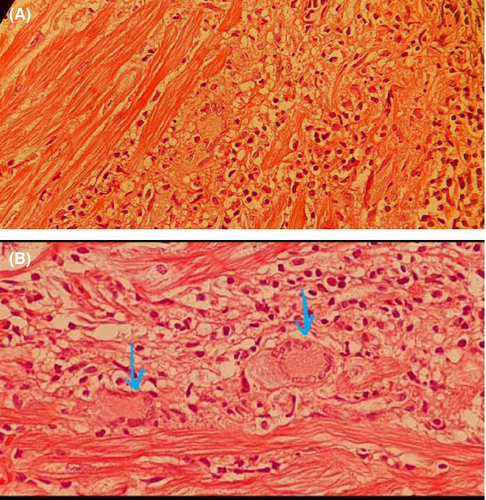
The microscopic examination of tissue demonstrated the myocardial fibers with multiple foci of mixed infiltration of inflammatory cells including lymphocytes, eosinophils, macrophages, and multinucleated giant cells associated by focal myocyte damage and areas of fibrosis mainly in subendocardial areas compatible with active myocarditis highly suggestive for GCM (Figure 3).
Immediately after reporting the pathology examination, a combined immunosuppressive therapy with IV pulse of methylprednisolone (500 mg for 3 days) and intravenous immune globin (IVIG [500 mg/kg for 3 days]) was prescribed for the patient. The IV pulse therapy with methylprednisolone was followed by 1 mg/Kg oral prednisolone, which was tapered by 5 mg daily. The tablet of mycophenolate mofetil 1000 mg, 2 times daily got also started for her. The oral tacrolimus, 1 mg two times daily got also started for her and adjusted to achieve the goal for a 12 h trough whole blood level of 10–15 ng/ml for her first 6 months.
On the third day of treatment, the patient's clinical condition and symptoms were remarkably improved and we could decrease and stop the IV inotrope and change the diuretic to oral doses. At the end of the week, her echocardiographic findings were significantly improved, LVEF increased to about 30%, TAPSE to 14 mm and RV sm to 8 cm/s.
The hospital course was uneventful, and she had no arrhythmias and/or hemodynamic deterioration. Reviewing the 48-hour ECG Holter monitoring showed no abnormalities in terms of arrhythmias and/or atrioventricular block and the patient was discharged after a week with a good clinical condition a normal kidney and liver function test, an NT-Pro BNP of 1580 pg/ml and a normal CTnI. A week later, the patient continued to be free of symptoms and her new echocardiography showed further improvement; mild LV enlargement with an EF to 40%, the apical segment of LV was still severe hypokinetic. Mild to moderate MR, near normal RV size and function with a TAPSE of18 mm and RVsm of 11 cm/s and mild TR and IVC diameter of 17 mm with good respiratory collapse.
The prednisolone daily dose was tapered to 30 mg, 2 grams daily dose of mycophenolate mofetil and tacrolimus was continued for her. At the end of 6th month of treatment, she had no clinical symptoms, the echocardiography demonstrated the same findings, so the dose of oral prednisolone was reduced to 10 mg/day. On the 3rd and 6th month of follow-up, her NT pro BNP level was 19.5 pg/ml and 30 pg/ml, respectively.
3 DISCUSSION
COVID-19 may present with cardiovascular manifestations and complications. Although currently it has been considered as an established etiology of myocarditis, there is no clear recommendation and consensus regarding the best clinical approach and the management of myocarditis following COVID-19.7-9, 11-14
Here, we reported a case of GCM following COVID-19 infection. Recently, Blagova et al. have reported a cardiomyopathy that was rapidly progressed to a sudden cardiac death following COVID-19 in a patient with a history of chronic adrenal insufficiency and myocardial disease with a final diagnosis GCM.10 As far as we researched, our case seems to be the first case report of GCM following COVID-19 in a patient with a background of autoimmune disorder, without any cardiac problem who was successfully treated with immunosuppressive therapy.
The etiology of GCM is unknown; however, an association with autoimmune diseases has been suggested. Many GCM cases are patients with immunological based diseases, such as thymoma, myasthenia gravis, dermatomyositis, thyroiditis, and pernicious anemia.1, 3, 4, 15-17 This association has prompted an autoimmune hypothesis for the etiology of GCM. Our patient had a history of an autoimmune dermatologic and thyroid disease.
It has been suggested that patients with chronic and long-term lichen planus (LP) dermatitis particularly those with erosive LP may have arterial stiffness secondary to autoimmune endothelial dysfunction and should be followed up in terms of cardiovascular disorders. However, the combination of GCM with autoimmune dermatologic including lichen planus has not been described yet.18, 19
Another issue which should be considered in patients with LP dermatitis is its differential diagnosis with sarcoidosis. In a case series, Goldberg et al have suggested that the LP can induce epidermotropic multinucleated giant cell inflammatory response and sarcoidosis with systemic involvement should be considered and worked up in these patients.20
Cardiac sarcoidosis (CS) could be considered as differential diagnosis of our patient. However, although cardiac sarcoidosis and giant cell myocarditis can be pathologically similar and pathological differentiation between the two may be difficult, cardiac sarcoidosis is not known to cause fulminant myocarditis and typically has a chronic course. Although there are a few reports of fulminant cardiac sarcoidosis, the treatment response of our patient could be in favor of GCM than CS.16, 21-24
Since the beginning of the COVID-19 disaster, physicians have encountered a variety of clinical manifestations and complications of this disease and it seems that we should expect a stranger behavior of this virus overtime. COVID-19 is associated with a hyperinflammation and activated humoral and cell-mediated immune response in human body, particularly in its severe forms.5, 9, 13, 25
Although the mechanisms of COVID-19-related myocardial injury are not completely understood, the role of severe inflammatory response with significant cytokines release, predominantly interleukin-6 (IL-6), has been recognized. Data from COVID-19 victims show mechanisms such as vasculitis, extended endotheliitis, lymphocyte infiltration, and direct injury of cardiomyocytes by binding spike protein of the SARS-CoV-2 to the membrane protein angiotensin-converting enzyme 2, which may be involved in acute cardiac injury and myocarditis following COVID-19.6-13, 26, 27
The presence of microvascular dysfunction accompanied with a myocardial inflammatory process may be an explanation for the subendocardial ischemic pattern noticed in cardiac magnetic resonance of patients with COVID-19 like what was seen in our patient and the case reported by Blagova et al.10
Although the clinical presentation of COVID-19 was mild, we believe that the autoimmune reactions following COVID-19 in our patient, who had a background of autoimmune abnormality, have caused GCM which was successfully controlled by immunosuppressive therapies. Choosing an optimal evidence-based immunosuppression regimen was challenging in this setting.
We opted to employ a combined immunosuppressive therapy utilizing IVIG, corticosteroid, tacrolimus, and mycophenolate mofetil for our patient based on earlier reports concerning the treatment of GCM and our center's previous experience with the treatment of GCM and recent COVID-19 infection.1, 2, 6, 14, 15, 17
However, several questions remained unanswered which will be clarified over time. First, as there are several reports of acute myocarditis following COVID-19 vaccination, which of the COVID-19 vaccines would be safe in patients who had myocarditis following COVID-19 infection, particularly those with inflammatory myocarditis such as GCM.28, 29 Secondly, the necessity of further systemic work up including FDG-PET or CMR and/or repeated EMBx to exclude CS or for prognostication. Third, what would be the correct surveillance for electrophysiologic complications in this patient? Despite the fact that our patient never developed arrhythmias or atrioventricular block, her LVEF increased to 40% and up to now she remains asymptomatic, considering the expert's recommendations30 we advised her for ICD implantation for the primary prevention.
In conclusion, as far as we investigated and researched, there is only one case report other than our case regarding the development of GCM following COVID-19. It seems that the development of myocarditis in COVID-19 would be independent of the severity of the illness. The current report supports the role of autoimmunity and immunosuppressive therapy in COVID-19 myocarditis including the inflammatory ones such as GCM. Fortunately, the currently recommended immunosuppressive therapies for GCM seem to be helpful in similar cases. Further investigations are needed to determine the appropriate diagnostic and prognostic approach in patients with this type of myocarditis and necessity of surveillance regarding the myocardial inflammatory disorders in known cases of autoimmune diseases who have COVID-19 infection without cardiac injury.
AUTHOR CONTRIBUTIONS
Afsaneh Amiri MD (First Author) contributed to idea for report, drafting the manuscript, and served as one of the members of patient's management team. Golnaz Houshmand MD, FSCMR involved in reporting the patient's CMR findings and drafting the cardiac MR section. Sepideh Taghavi MD, Mona Faraji MD, and Monireh Kamali MD involved in collecting the data for case report and served as one of the members of patient's management team. Nasim Naderi MD, FESC (corresponding Author) involved in editing and final drafting the manuscript and served as the main physician of patient's management team. Each author has made an independent material contribution to the work and this paper has not been published or submitted in any other journal and all authors have read and approved the manuscript to be published in Clinical Case Report journal and have no disclosures to admit.
ACKNOWLEDGMENTS
We would like to thank you our colleagues Dr. Ali Hesami (Mostoufi Lab.) for prompt evaluation and report of endomyocardial biopsy specimen as well as Dr. Nickulas Austin for the language editing of the manuscript.
CONFLICT OF INTEREST
The authors disclose that this study has no relationship with industry and financial associations and no competing interest has been declared. All of the authors of this case report are independent investigator and do not have any relationship with the activities of the Iran government.
ETHICAL APPROVAL
It was approved to be reported by the ethics committee of Rajaie Cardiovascular Medical and Research center with ethical code of IR.RHC.REC.1400.066.
CONSENT
This case report is about a case with a diagnosis of giant cell myocarditis following COVID-19 infection in a lady with a history of autoimmune disorders. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.