Invasive ductal carcinoma of breast with neuroendocrine differentiation: A case report
Abstract
Primary invasive breast carcinoma with neuroendocrine differentiation is an uncommon presentation. We hereby report a case diagnosed as invasive ductal carcinoma with neuroendocrine differentiation in a 52-year-old female patient who presented with a painless right breast lump.
1 INTRODUCTION
Invasive carcinoma with neuroendocrine features is a rare entity that accounts for 2%–5% of all invasive carcinoma of the breast.1 It has comparable histological features with other neuroendocrine tumors of the gastrointestinal tract, pancreas, and bronchopulmonary system with different percentages of neuroendocrine markers. The diagnosis of the neuroendocrine tumors of the breast is confirmed based on immunohistochemistry markers, which is not routinely done for the breast tumors, so, the exact incidence is still unknown.2 Some literature report that neuroendocrine tumors are seen in up to 30% of invasive carcinoma commonly associated with solid papillary carcinoma, invasive ductal carcinoma, and mucinous carcinoma of the breast.3 A novel hypothesis states that the tumor arises from differentiating neoplastic stem cells into epithelial and endocrine lines as a consequence of the unusual differentiating event during carcinogenesis.4 The neuroendocrine markers such as chromogranin A or B and synaptophysin are generally positive for these cancers.5 Here, we present a case of a rare variant of the invasive ductal breast with neuroendocrine differentiation.
2 CASE REPORT
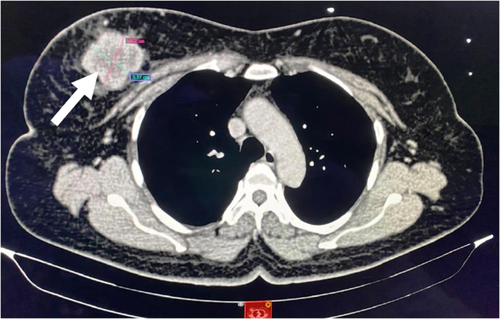
A 52-year-old female patient presented with a painless right breast lump for a duration of 1 week. On local examination of the breast, the mass measured 6 cm × 4 cm and was located at 12 o'clock position. The overlying skin and nipple-areolar complex were unremarkable. Axillary lymph nodes were not palpable. Initially, the patient was evaluated with a mammogram, which showed a lobulated dense mass measuring 49 mm × 48 mm situated in the upper inner quadrant of the right breast (BI-RADS category 4a). Further evaluation with fine needle aspiration cytology (FNAC) from the breast mass showed cytomorphological features of ductal carcinoma. Computed tomography (CT) scan of the chest revealed heterogeneously enhancing mass measuring 4.4 cm × 4.3 cm in the upper inner quadrant of the right breast with surrounding perilesional fat stranding and few right axillary lymph nodes with maintained fatty hilum but with thick eccentric cortex—likely to be metastatic (Figure 1).
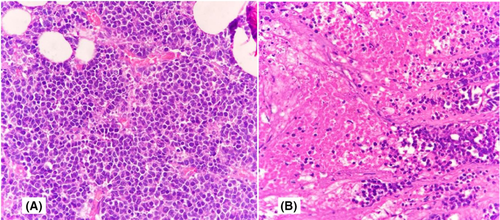
Later, a USG-guided tru-cut biopsy was performed, and a diagnosis of ductal carcinoma was made. The patient underwent right breast mastectomy with frozen section examination of right axillary sentinel lymph nodes. On frozen sections examination, four lymph nodes identified were uninvolved by tumor deposits. Gross examination revealed a unifocal tumor located in the upper inner quadrant of breast. The greatest dimension of the tumor was 4 cm. Histopathological examination revealed monomorphic round tumor cells arranged in diffuse sheets and pseudorosettes. These tumor cells have round to oval nuclei, powdery chromatin with scant cytoplasm. Mitosis and areas of necrosis were noted (Figure 2). Further, immunohistochemistry performed showed tumor cells positive for cytokeratin (CK), focally positive for insulinoma-associated protein 1 (INSM-1), cluster of differentiation 56 (CD56), and negative for transcription factor GATA3, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (Her2 Neu) with nuclear protein (Ki67) proliferation of about 40% (Figure 3).

Based on the cumulative information obtained from all the diagnostic procedures including histopathology and immunohistochemistry, the final diagnosis of invasive carcinoma with neuroendocrine differentiation was considered. The pathological stage was pT2(sn)N0. The postoperative period was uneventful. The patient has received 4th cycle of chemotherapy with carboplatin and docetaxel 3 weekly (L1C4 completed) to date. USG of breast, chest X-ray, USG of abdomen and pelvis, and metabolic panel were performed at 4 months of follow-up, which showed no evidence of recurrence of the disease.
3 DISCUSSION
- Well-differentiated,
- Poorly differentiated neuroendocrine/ small cell carcinoma, and
- Invasive breast carcinoma with neuroendocrine differentiation.
There is no specific radiological or clinical sign to diagnose the neuroendocrine tumor. Histopathological examination and immunohistochemistry are the only means of making a definitive diagnosis. Grossly, the tumor is usually yellow colored with a firm consistency and multilobulation.8 In this case, we found the tumor to be a gray-white lobulated structure. Histologically, the tumor shows similar features as neuroendocrine tumors of the gastrointestinal tract with nuclear palisading, cellular monotony, loss of cell cohesion, pseudorosette formation, abundant eosinophilic cytoplasm, and nuclei with stippled chromatin.9 Since the microscopic features are inconsistently present, IHC markers such as chromogranin A or B, synaptophysin, neuron-specific enolase (NSE), CD-56, Ki-67, Leu 7 (CD-57), INSM-1 are necessary for the diagnosis.10 INSM and CD-56 were focally positive, and Ki-67 is around 40%.
This tumor should be differentiated from metastatic neuroendocrine tumors from extramammary sites as they show features similar to other primary tumors of the breast such as lobular carcinoma and invasive ductal carcinoma in situ.2 Some markers such as CK-7 and caudal-type homeobox-2 (CD X-2) are useful to differentiate from GI neuroendocrine tumors. Usually, the neuroendocrine breast carcinomas are positive for CK-7 and negative for CDX-2, whereas in gastrointestinal neuroendocrine tumors, CK-7 is negative and CDX-2 is positive.11
Invasive breast carcinoma with neuroendocrine features is treated similar to other invasive breast carcinomas. The primary treatment option is surgery depending upon the stage and location of the tumor.1 The combination of mastectomy, axillary lymph node dissection, and metastasectomy is generally performed for surgical treatment. Like other breast carcinomas, they are treated by chemotherapy and radiotherapy. Immunohistochemistry helps to determine the type of adjuvant therapy.8
The prognosis of breast carcinoma with neuroendocrine features generally depends upon the factors such as age of the patient, axillary lymph node involvement, clinical-stage, and hormonal receptors.12 Neuroendocrine differentiation was said to be an independent adverse prognostic factor according to the multivariant analysis based on (Surveillance, Epidemiology, and End Results Program) SEER database.5 In a retrospective comparative study by Zhang et al.,10 the breast carcinoma with neuroendocrine features showed a poorer survival rate, lower recurrence-free survival rate, and a high rate of distant metastasis. Some literature reported that these tumors have a similar prognosis with invasive breast carcinoma when the clinical stage and grade of the tumors are compared.13, 14
4 CONCLUSION
The histomorphology and the immunohistochemical study of the neuroendocrine markers are the basis for the definitive diagnosis of invasive breast carcinoma with neuroendocrine differentiation. But the combined approach with clinical assessment and radiological images aids in the diagnosis. The current diagnostic methods and treatment protocols are similar to other invasive breast carcinomas. As it has a poorer prognosis than invasive carcinoma of the breast without neuroendocrine tumors, it is important to make the early diagnosis of the neuroendocrine component. Hence, larger studies are needed to better understand the biological behavior of invasive breast carcinoma with neuroendocrine differentiation.
AUTHOR CONTRIBUTION
KSA was involved in counseling, treatment of the patient, and collection of CT-scan chest image. HPD and MS examined and interpreted the pathology. VA, KN, AM, and MS collected the required case information, images, slides, and reports and contributed to writing manuscripts. VA, AM, KN, ASD, and HPD reviewed the literature and contributed to both writing and editing the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The corresponding author acknowledges the Radiology department for the CT scan images and Core Diagnostics Pvt. Ltd., for immunohistochemical analysis.
CONFLICT OF INTEREST
The authors declare that there is no potential conflict of interest with respect to the research, authorship, and /or publication of this article.
ETHICAL APPROVAL
The hospital research board (HRB) of Nepal Cancer Hospital and Research Center, Harisiddhi, Lalitpur, Nepal, provided approval.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.