The use of a simultaneous fascia lata-free flap with cochlear implantation after radical Mastoidectomy
Abstract
Patients undergoing cochlear implant after prior radical mastoidectomy are at increased risk of device infection requiring device explant. Various techniques including two-stage operations have been used. We report the novel technique with use of a vascularized fascia lata free flap for a patient undergoing cochlear implantation with radical mastoidectomy.
1 INTRODUCTION/BACKGROUND
Cochlear implantation is now the standard of care for patients with severe to profound sensorineural hearing loss in whom hearing aids are no longer beneficial. Patients undergoing cochlear implantation (CI) after prior radical mastoidectomy are at increased risk of device infection, a potentially devastating complication requiring device explant. The open mastoid cavity can lead to extrusion of the electrode and device failure. There is also a concern for increased risk of infection leading to labyrinthitis or meningitis given the altered anatomy and underlying chronic ear disease. Various techniques including two-stage operations have been used in patients with radical mastoidectomy anatomy in order to mitigate those risks. CI in patients who have undergone previous canal wall down (CWD) mastoidectomy can be a challenging problem.
There exist several strategies in technique to overcome the challenge of CI in patients with previous CWD mastoidectomy. The particular challenge faced when performing CI in a patient who has previously undergone CWD mastoidectomy revolves around the higher rate of electrode or device extrusion. Strategies to overcome this problem include soft tissue coverage of the electrode, overclosure of the external auditory meatus (EAM) with and without mastoid obliteration or eustachian tube (ET) plugging, and reconstruction of the posterior external auditory canal wall.1-5 The advent of several new techniques in recent years has met with varying degrees of success. Other options include the use of the middle fossa approach to the cochlea, while other surgeons have advocated for the partial obliteration of the mastoid cavity using tragal cartilage and bone paté with success.6-8
Free flap reconstruction has previously been reported in patients with CWD mastoidectomy with a history of osteoradionecrosis and profound hearing loss.9 Free flap reconstruction is an excellent option to placed vascularized tissue in an area that has often been subject to chronic inflammation. The team approach for free flap reconstruction allows for an excellent option for cochlear implantation in a patient who has previously undergone CWD mastoidectomy. Surgical planning is key to this approach.
Here, we report the novel technique of a single-stage procedure for cochlear implantation in a patient with canal wall down mastoidectomy anatomy with the use of a vascularized fascia lata-free flap. This case follows the clinical course of a previously healthy man with a history of left CWD mastoidectomy and eventual combined transotic, middle fossa, and suboccipital approach on the left side, with complete removal of the cochlea and vestibule. He also had a history of right CWD mastoidectomy with a large meatoplasty previously performed. He had a history of bilateral profound sensorineural hearing loss. This report describes the team approach to free flap reconstruction with fascia lata-free flap reconstruction for simultaneous coverage and obliteration of CWD mastoidectomy in a patient undergoing cochlear implantation.
2 CASE REPORT
A 61-year-old male patient presented to us with right moderately severe to profound sensorineural hearing loss (SNHL) and left profound SNHL. He had a word recognition score of 48% in the right ear and no hearing in the left. He had a history of right CWD mastoidectomy for cholesteatoma performed 20 years prior. He also had extensive surgery on the left side 39 years ago. A CT scan was obtained confirming the right CWD mastoid cavity, but also evidence of a combined transotic, middle fossa, and suboccipital approach on the left side, with complete removal of the cochlea and vestibule. It was unclear what the exact pathology was on the left side requiring such surgery. On physical examination, his bilateral mastoid cavities were stable without active infection, with an intact tympanic membrane on the right side.
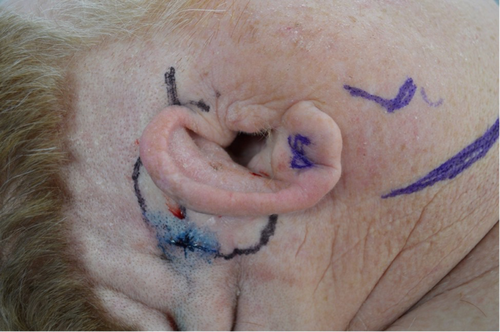
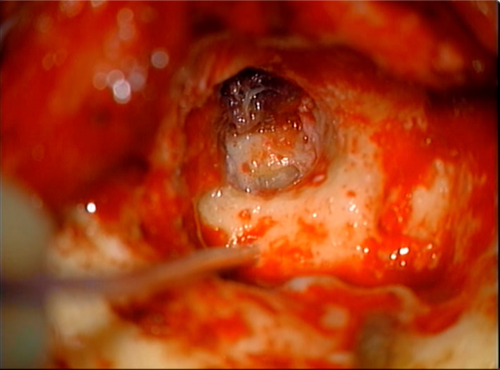
Given the extensive surgery on the left side with prior removal of the vestibule and cochlea, right-sided cochlear implantation was pursued. However, due to his previous right-sided ear surgeries, he had limited soft tissue coverage over the anticipated implant site. The patient had a wide meatoplasty which would make creation of a blind sac difficult (Figure 1). Plans for fascia lata-free flap based on the descending branch of the lateral femoral circumflex artery for coverage of the implant and obliteration of the mastoid and middle ear cavities were made (Figures 2, 3).10
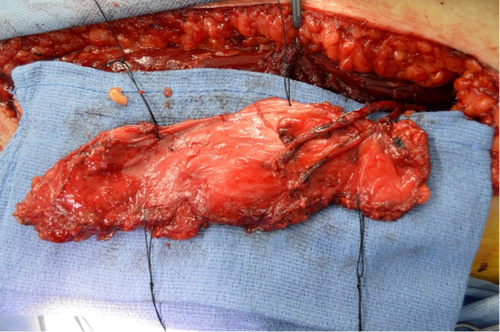
Intraoperatively, the right ear canal blind sac was created but noted to be tenuous. The squamous epithelium lining the bony canal and tympanic membrane was removed. The previous mastoid cavity was revised, and all mucosa was then removed from the mastoid and middle ear (Figure 4). The eustachian tube was plugged with muscle. The receiver stimulator was then placed in a tight subperiosteal pocket, and the electrode was inserted through the round window with full insertion (Figure 5).
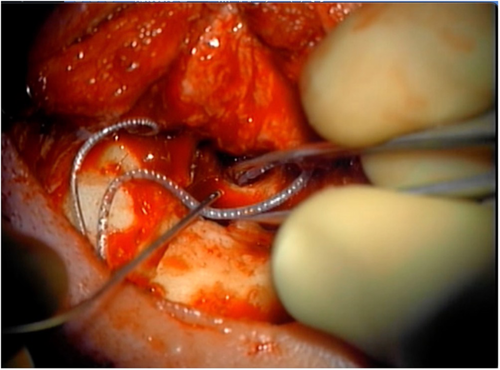
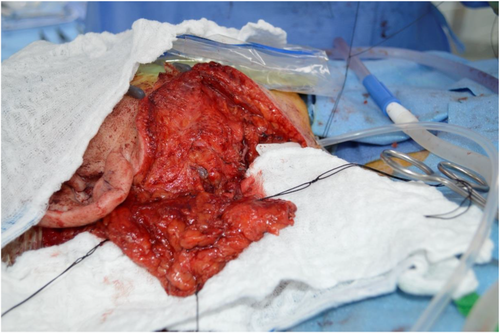
Following implantation, a left fascia lata-free flap was harvested as described above and anastomosed to the right facial artery and vein.10 This vascularized fascial flap was then used to obliterate the middle ear and mastoid bowl, provide coverage to the cochlear implant receiver stimulator and electrode, and reinforce the blind sac closure of the ear canal (Figure 6). The patient was kept in the hospital and discharged in good condition on post-operative Day 3.
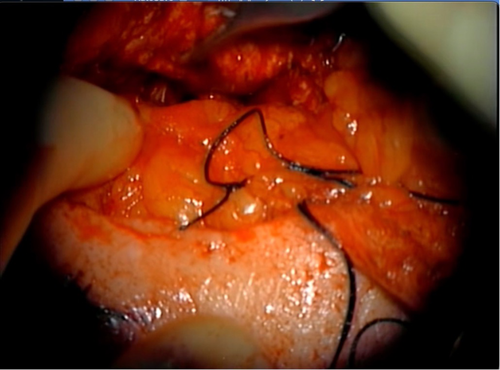
The patient returned for follow-up in the office 2 weeks later for device activation. The implant was in good position with healthy overlying skin and a well-healed blind sac. At the time of writing, it has been 7 months since surgery, and the patient has continued to improve with his cochlear implant with no signs of wound breakdown, infection, or recurrent cholesteatoma.
3 DISCUSSIONS/CONCLUSIONS
Chronic otitis media and cholesteatoma leading to radical mastoidectomy present a unique challenge for cochlear implantation. Cochlear implantation has become the standard of care for patients with severe to profound sensorineural hearing loss in whom hearing aids are no longer beneficial. Patients who have profound sensorineural hearing loss and a previous history of CWD mastoidectomy present a unique challenge. These patients are at higher risk for device infection, extrusion, and possible need for explantation. This is a result of exposure of the electrode and device to the environment via the previously created mastoid cavity. Several techniques have been developed to mitigate this risk. Options include EAC overclosure or blind-sac closure of the EAC. Other options include partial obliteration of the mastoid cavity, middle fossa approach to the cochlea, and the use of a two-stage procedure. Ugo Fisch described subtotal petrosectomy in 1965. This technique has been reported by several authors for cochlear implantation in patients with CWD mastoidectomy.6
The risk of CI in patients with previous CWD mastoidectomy consists primarily of the risk of device infection and need for subsequent explanation and the risk of electrode or device extrusion. Other challenges inherent to implantation in patients who have previously undergone CWD mastoidectomy include risk of infection and meningitis. This is in addition to the risk of electrode and device extrusion. Several techniques have been developed to help mitigate this risk.
Overclosure of the EAC is a common strategy for patients with a prior CWD mastoidectomy undergoing cochlear implantation. However, studies have shown an increased risk of complications and dehiscence in patients with a large meatoplasty.3 The complication rate for patients who do not undergo overclosure of the EAC is much higher still. Undetected cholesteatoma is a possible risk for patients who undergo EAC overclosure. This is particularly problematic since it would be difficult to detect with a masked cavity.
A technique that has been developed to deal with the risks and complications of EAC overclosure include a technique of partially obliterating the mastoid cavity. There are various techniques to accomplish this such as the use cartilage and bone paté.6 There still other options as well for avoiding EAC overclosure. These other options include reconstruction of the posterior canal wall and middle fossa approaches.7, 8 Two-stage procedures have been used as an option for this patient population. However, there are disadvantages and risks as outlined above.
Other techniques described include maintaining an open mastoid cavity with soft tissue coverage of the electrode, posterior canal wall reconstruction, and mastoid obliteration with ET plugging and blind sac closure of the EAM. There is potential to better monitor for recurrence of disease by maintaining an open cavity. The technique described in these cases includes partial obliteration of the mastoid bowl with bone pate and cartilage, or the use of pedicled muscle or periosteal flaps to provide soft tissue coverage to the electrode as it passes through the mastoid cavity.3, 6, 11 Caution is needed with future mastoid bowl cleanings in this patient population.
Mastoid obliteration with ET plugging and EAM closure is a commonly described technique (Video S1). This can be performed as a single or two-stage operation. Single-stage procedures are only considered in a stable, dry ear. Two-stage operations are performed when there is active disease or high suspicion for recurrent disease. Various materials have been described for obliteration including bone pate, muscle, or abdominal fat. Benefits of mastoid obliteration with ET plugging and EAM closure include decreased rate of extrusion.3 However, it does prevent monitoring for recurrent disease. Given the nature of the chronic disease, long-term follow-up is necessary to prevent unrecognized recurrent disease.12 CT follow-up has been proposed to monitor for recurrence; however, the sensitivity is low in detecting recurrent cholesteatoma.11, 13, 14 Another difficulty with this approach is maintaining EAM closure in the setting of previous large meatoplasty. Common complications in these procedures included dehiscence of the blind sac.3, 6, 14-16 This was anticipated in our case and countered with the free flap reinforcement.
The use of fascia lata-free flap allowed for a single-stage procedure for this patient. The free flap allowed for simultaneous coverage for the obliterated mastoid cavity and good soft tissue reinforcement for blind-sac of the EAC even though the patient had large meatoplasty (Video S2). The use of the fascia-free flap in this case greatly lessened the risk of dehiscence of the blind-sac EAC. We also stripped all mucosa from the middle ear space and packed the eustachian tube as well as part of the blind-sac procedure. The use of a simultaneous free flap allowed for single-stage procedure for both cochlear implantation and reconstruction of the obliterated mastoid cavity and blind-sac EAC.
The fascia lata-free flap was a great option for vascularized tissue to reinforce and greatly decrease the risk of dehiscence of the blind-sac EAC in this patient with prior CWD mastoidectomy and large meatoplasty. This is an excellent option for appropriately selected patients with canal wall down mastoidectomy anatomy undergoing cochlear implantation. Having robust soft tissue coverage and reinforcement as well as close follow-up will provide the best outcomes: a successful implantation.
AUTHOR CONTRIBUTIONS
J May contributed to research idea, writing and editing of article, and served as a submission author. J Kerr involved in writing of article. Ja May involved in video editing and preparation of figures and photographs, and editing of article. P Tassone contributed to research idea, photographs of case, and editing and feedback. A Rivera contributed to research idea, video recording, and editing of article and feedback.
ACKNOWLEDGMENTS
None.
CONFLICTS OF INTEREST
No conflicts of interest to report.
ETHICAL APPROVAL
Project was IRB approved by the University of Missouri IRB.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.