Noninvasive treatment of two deeply invasive cutaneous squamous cell carcinomas of the midface located close to the orbits with intensity-modulated arc therapy: A case report
Abstract
Definitive radiotherapy is a curative and noninvasive treatment modality for cutaneous squamous cell carcinomas of the midface when surgery has an impact on function and cosmetics. Volumetric modulated arc therapy provides optimal dose coverage for complex-shaped tumors without compromising adjacent organs at risk.
1 INTRODUCTION
Definitive radiotherapy is a curative treatment modality in patients with cutaneous squamous cell carcinomas (cSCC) and is frequently used in anatomical areas such as the midface when surgery compromises function and/or cosmetics.1, 2 Volumetric modulated arc therapy (VMAT) is an advanced radiotherapy technique that provides conformal dose coverage for complex-shaped tumors with reduced dosage to the adjacent critical organs at risks such as the globe of the eyes or lacrimal glands.3, 4 This case shows VMAT planning of an elderly patient with two large ulcerated cSCC in the midface with proximity to sensitive organs at risk. We report a complete response to definitive radiotherapy after granulation tissue formation with a favorable cosmetic result.
2 CASE HISTORY
A 91-year-old White woman presented to the emergency department of the University Hospital of Regensburg with a deeply invasive 3.1 × 3.1 × 3.0 cm tumor on the right cheek (cT3 cN0 cM0, stage II) and a 1.5 × 1.5 × 1.1 cm tumor of the left nasal wing (cT2 cN0 cM0, stage II), classified using the Union for International Cancer Control (UICC) staging system (8th edition). Figure 1 shows the patient at the time of presentation. The patient lived in a care facility and was dependent on nursing care (Karnofsky Performance Score: 60). The patient had good cognitive performance and neglected pain or discomfort. The patient suffered from arterial hypertension and had a femoral neck fracture several years ago. She used a walker every single day. The patient had physician reported progressive presbyopia and required reading glasses. The family of the patient reported that the tumor had begun as a small lesion 1 year ago and expanded to its measured size at the time of presentation. The reason for the current presentation was tumor bleeding due to an accidental fall from a chair in the care facility. The patient had no history of immunosuppression or family history of cancer. The patient was presented with her son to the Department of Otolaryngology—Head and Neck Surgery of University Hospital Regensburg. Biopsies of the lesions revealed two moderately differentiated cSCC. There was no evidence of metastatic spread to the neck. The patient was offered operative procedures for both tumors with skin grafting of the lesion of the right cheek. However, the patient preferred a noninvasive treatment and was presented to the Department of Radiation Oncology. We performed planning computed tomography (Aquilion LB, CANON MEDICAL SYSTEMS CORPORATION) and used a thermoplastic head mask for immobilization and fixation. The CT scan showed the two deeply invasive tumors on the right cheek and on the left nasal wing with surrounding tissue infiltration (Figure 2). The planning target volume (PTV) was defined as the volume of the tumors plus microscopic extension with an additional margin of 1 cm. A 0.5 cm thick bolus material was placed over both tumors to ensure sufficient surface doses. Our institution used Monaco external beam-planning software (Version 5.11, Elekta) for contouring and VMAT planning. The treatment was performed with a modern linear accelerator and multi-leaf collimators of 5 mm (Elekta Synergy Agility). We performed VMAT by 6 megavoltage photons and daily 558.7 monitor units. The physical treatment plan consisted of two double arcs. Patient positioning during treatment was verified by three-dimensional cone-beam CTs. Figure 2 shows CT planning axial slices through the tumor with isodoses from the original radiotherapy treatment plan. 64Gy at 2Gy per daily fraction was prescribed to the PTV (biologically effective dose, BED 10 76.8Gy; for cSCC α/ß was considered to be 10Gy) and dose constraints of organs at risk were set to the treatment planning system. Organs at risk were the globe of the eyes, lenses, lacrimal glands, optic nerves, retina, optic chiasm and brainstem. The objective was to deliver a prescribed dose of 64Gy in 32 fractions to at least 95% of the planning target volume. The treatment was aimed to reduce doses to organs at risk as much as possible without compromising the coverage of the planning target volume. The doses to the organs at risk were evaluated accordingly to the mean dose and the maximal dose (D max) which was defined as the dose to a volume of 0.02 cm3 (D 0.02 cm3). We planned with a D max of <54Gy and a mean dose of <35Gy to the globe of the eyes, a D max of <10Gy to the lenses and a D max of <40Gy and a mean dose of <26Gy to the lacrimal glands. We put high priority on the D max of the right lacrimal gland, lens and globe of the eye because these organs were very close to the planning target volume. Dose distributions and planning objectives in planning target volume and organs at risk were checked by dose–volume histograms and 3D isodose lines. We spared normal tissue from the higher radiation doses with steep dose gradients and met all dose constraints except for the right lens (D.02 cm3 of the right lens = 16Gy).
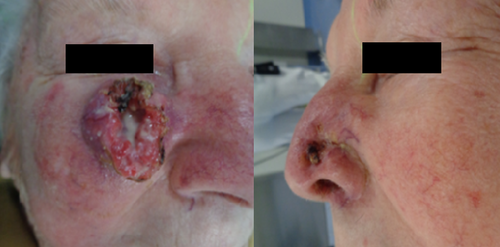

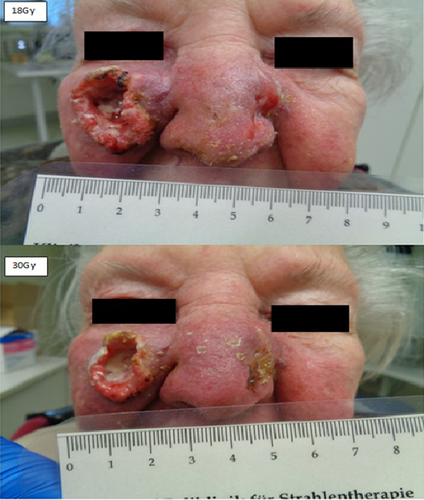
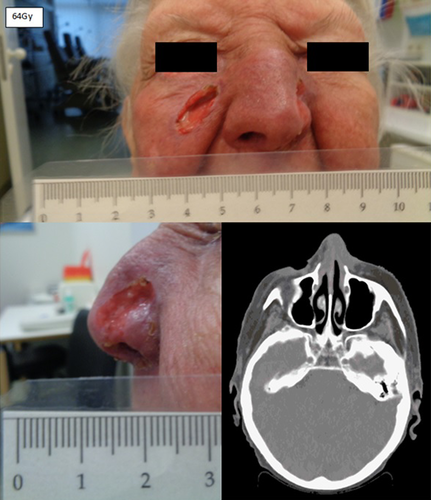
Figure 3 shows the clinical appearance after 9 fractions of radiotherapy (18Gy, BED 10 21.6Gy) and 15 fractions of radiotherapy (30Gy, BED 10 36.0Gy). We found exudation and central necrosis of the inner portion of the tumor. The wound exudation was greatly reduced in the course of radiotherapy and healing and re-epithelialization proceeded from the wound edges. Figure 4 depicts a clear regression of the initial tumors on the final day of radiotherapy (64Gy, BED 10 76.8Gy) and a beginning second-intention granulation. CT scan showed a clear remission of the initial tumors on the final day of radiotherapy. In summary, we found dermatitis CTC grade 2 of the skin and keratitis CTC grade 1 of the eyes, using the Common Terminology Criteria for Adverse Events (CTCAE v. 4.0). The patient was examined daily during treatment to prevent infection and to care for the skin, eyes and nasal mucosa with wound and healing ointment. The patient reported symptoms of conjunctivitis and irritated eyes but was painless at any point during treatment. No bleeding or infection occurred. The wound on the cheek and nose healed rapidly after termination of radiotherapy by secondary intention. We recommended intensive care of the eyes and skin with wound and healing ointment. The patient was highly satisfied with the cosmetic result after definitive radiotherapy and she reported that her vision was normal with her standard pair of reading glasses. The patient is now at a 1-month follow-up without clinical evidence of tumor recurrence.
3 DISCUSSION
Surgery is the preferred treatment modality for most patients with cSCC. International guidelines consider primary (or definitive) radiotherapy as a curative treatment alternative for cSCC of the midface when surgery could have an impact on form, function and/or cosmetics. Definitive radiotherapy avoids the surgical morbidity and requirement for reconstruction and is, therefore, a useful alternative to surgery. Older or comorbid patients are also often better treated with definitive radiotherapy.1, 2, 5 Moreover, definitive radiotherapy leads to high local control rates similar to surgery. Although there is no prospective randomized controlled trial comparing the effectiveness of definitive radiotherapy and surgery in tumor control and overall survival, retrospective studies show a long-term tumor control without severe acute or late side effects by definitive radiotherapy.5-7 A systematic review and pooled analysis of observational studies of Lansbury et al.8 reported that studies with a mean follow-up period between 2 and 5 years had an average recurrence rate of 6.1% (95% confidence interval 2.2–11.7) after definitive radiotherapy.
The patient of the case report was treated with conventional fractionation (2Gy/fx with BED 10 76.8Gy) in accordance with international recommendations.1, 2 VMAT and daily nursing care contributed to a safe treatment and a favorable cosmetic result after secondary tissue granulation. Pretreatment imaging and VMAT allow high-precision delivery of radiotherapy to the tumor while sparing normal tissue. Critical organs at risk can be shielded using multi-leaf collimators and steep dose gradients without compromising the coverage of the planning target volume.9 Incomplete resections or mutilating surgery of cSCC in the face are not justified because cSCC are highly radiosensitive tumors.1 Even large tumors can be cured with definitive radiotherapy. Treatment of cSCC at the earliest stage is however recommended. Early treatment improves disease control and minimizes cosmetic detriment.2 Despite advances in radiotherapy, some patients receive no or delayed treatment. Clinicians should be aware of these highly radiosensitive tumors because early treatment results in the best cosmetically and functionally result. We tried to achieve an optimal balance between tumor control and functional preservation of organs at risk. Complete avoidance of toxicity to organs at risk is not possible. We met all dose constraints except for the right lens because the planning target volume was immediately adjacent to the right lens. However, we have to note that radiation-induced cataracts can be easily surgically corrected.
4 CONCLUSIONS
Definitive radiotherapy of cSCC in anatomically sensitive areas of the face is feasible with clinical acceptable toxicity for organs at risk and represents a curative and noninvasive alternative when surgery compromises function and aesthetics.
AUTHOR CONTRIBUTIONS
Isabella Gruber has first authorship and is responsible for acquisition of data, analysis and writing the manuscript. Karin Weidner is responsible for acquisition of all doses of the radiotherapy treatment plan. Oliver Koelbl revised the manuscript critically for content. All authors gave approval of the final version.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL. WOA Institution: UNIVERSITATSKLINIKUM REGENSBURG Consortia Name : Projekt DEAL
CONFLICT OF INTEREST
Isabella Gruber, Karin Weidner and Oliver Koelbl declare that they have no competing interests regarding this case report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The institutional ethics committee of University of Regensburg approved the case report (Number: 22-2908-104).
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.




