Pregnancy management for a woman with extensive vulvar and pelvic malformations caused by Klippel–Trénaunay syndrome
Abstract
Klippel–Trénaunay syndrome (KTS) is a rare congenital disorder defined by a triad of capillary malformation, venous malformation, and soft tissue or bone hypertrophy most commonly affecting unilateral lower limbs. Due to the rarity of KTS, evidence-based guidelines for the management of pregnancy in people with KTS are still lacking. A 34-year-old woman (gravidity 1; parity 0) presented at 25 weeks of gestation with malformations of the right side of her body. The extent of the KTS affecting the vulva, pelvis, and right leg was remarkable. As the prenatal MRI showed massive vascular malformations of the pelvis and vulva, we performed an elective cesarean section to avoid severe perinatal hemorrhage during a vaginal delivery. Intraoperatively, we observed varices on the parietal peritoneum within the vesico-uterine pouch and the isthmocervical transition of the uterus, which were not identifiable in the preoperative MRI. Although KTS patients have been discouraged from pregnancy in the past because of a high risk for complications, successful and uncomplicated pregnancies are possible. For this purpose, we believe a multidisciplinary strategy that is crucial.
1 BACKGROUND
Klippel–Trénaunay syndrome (KTS) is a rare congenital disorder with an estimated incidence of between two and five people in 100,000, without any apparent sex predisposition.1, 2 The etiology and physiopathology of KTS remain indistinct but has been recently attributed to somatic mutations.3 Historically, KTS has been defined by a triad of capillary malformation, venous malformation, and soft tissue or bone hypertrophy most commonly affecting unilateral lower limbs.4 However, the severity of these components can vary from patient to patient.5
Due to the rarity of KTS, the evidence is limited to only a few case reports and retrospective studies dealing with severe complications during pregnancy in people with KTS. Hence, evidence-based guidelines for the management of pregnancy in such patients are still lacking. However, existing data indicate that patients with KTS are, for example, more likely to experience a postpartum hemorrhage (PPH) or deep vein thrombosis (DVT) during pregnancy or childbed.6-8
2 CASE PRESENTATION
A 34-year-old woman (gravidity 1; parity 0) was referred to our hospital at 25 weeks of gestation. She was diagnosed with KTS in her early childhood after her doctor noticed hypertrophy of the right lower leg and referred her to a specialized pediatric clinic. The patient does not know any other cases of KTS in her family or genetic predisposition for KTS.
Throughout her life, hemorrhoids and intravesical and rectal malformations caused recurrent bleeding—sometimes to an excessive extent. The patient also had dozens of vulvar and gluteal thromboses and thrombophlebitides, anal venous thromboses (particularly during childhood), and a DVT in her leg.
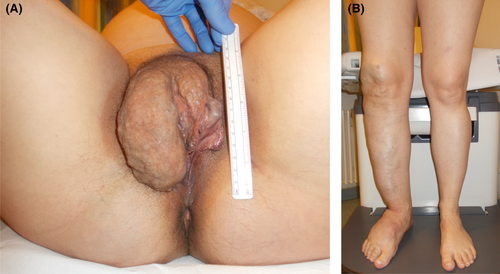
The extent of the malformations of the right side of her body, especially of the vulva, pelvis, and right leg, was significant (Figure 1A + B). In the clinical examination, these vascular malformations appear as soft, compressible lesions without hyperthermia or pulsation with a characteristic blue coloration of the overlying skin. Nevertheless, the massive hypertrophy of the right labia led to recurring treatments. Radiological embolization of the right obturator artery and the branches of the superficial external pudendal artery had not been successful.
After natural conception of her planned pregnancy, the patient's obstetrician managed prenatal care until 25 weeks of gestation, when the patient was admitted to our obstetrical outpatient clinic. The course of the pregnancy had been widely uneventful. Fetal ultrasounds conducted throughout the pregnancy showed adequate fetal growth and feto-maternal circulation without any pathological findings.
We initiated interdisciplinary counseling with specialists from the departments of angiology and vascular surgery, radiology, and hemostaseology from our tertiary care university medical center, Mainz. Because of the elevated risk of thromboembolism, we started a prophylactic anticoagulation therapy with low-molecular-weight heparin (LMWH) (Dalteparin 5000 IE once per day). In the last trimester, we increased the dose to twice daily.
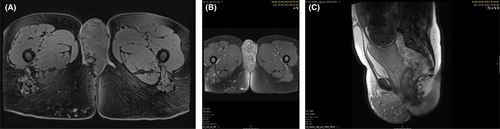
At 32 weeks of gestation, we performed an MRI of the lower abdomen and pelvis without contrast to facilitate optimal operation planning. Compared with previous imaging, our MRI results showed widely stable vascular malformations of the right labium majus, as well as in the pelvis dorsolateral to the cervix uteri, in the mesorectal adipose tissue, and in the left ischioanal fossa (Figure 2).
Based on these findings, and considering the high risk of perinatal bleeding, we discussed primary cesarean delivery (C-section) with the patient. On March 16, 2021, the patient underwent a primary C-section at 38 weeks and 3 days of gestation under permanent on-call service from the department of interventional radiology. Considering the elevated risk of bleeding, we performed the operation via longitudinal laparotomy under general anesthesia. LMWH therapy was paused the evening before surgery and continued 12 hours after the procedure at prophylactic dosage of one dose per day during the rest of her hospitalization.
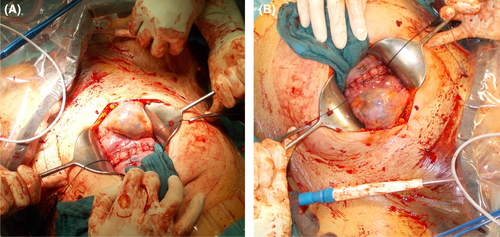
Despite a recent MRI describing no antero-uterine vascular malformations, we intraoperatively observed large varices on the parietal peritoneum within the vesico-uterine pouch and the isthmocervical transition of the uterus (Figure 3). Consequently, we chose a transverse section above the lower uterine segment. The rest of the operation proceeded without any further complications. The intraoperative blood loss was about 500 ml (Figure 4). The immediate postnatal period of the female fetus was without any pathological events and without any clinical signs of KTS (APGAR: 8/9/10; pH [umbilical artery]: 7.32; weight: 2610 g; length: 46 cm; head circumference: 34 cm).
Around 6 hours after the C-section, the patient showed a PPH with blood loss of approximately 1000 ml. After administering oxytocin and misoprostol to induce enforced uterine contraction, the bleeding suspended. Therefore, a diminished tonus of the uterus was the most probable cause of the PPH. No further interventions, such as surgical procedures or blood transfusions, were necessary.
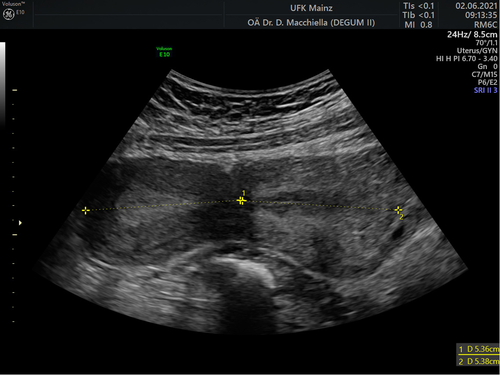
Over the following days, we observed an uncomplicated lochiostasis with neither signs of retention of placental tissue nor infection. The lochia dissolved after a combined oxytocin–methylergometrine infusion. We discharged the patient 6 days after delivery under continuous anticoagulation therapy with LMWH (Dalteparin 5000 IE once per day). During the patient's 6-week and 12-week follow-ups, the patient and her newborn showed a normal and physiological puerperium. However, we observed a prominent hourglass-shaped uterine cervix with a cervix-to-body ratio of 1:1 in the follow-up ultrasound, which had not been observed before pregnancy (Figure 5). The deformation led to no further consequences.
3 DISCUSSION AND CONCLUSIONS
We reported a rare case of pregnancy in a woman with KTS complicated by severe vulvar and pelvic vascular malformations, as well as a massive hypertrophy of the right leg. In the following sections, we will discuss several aspects of the case.
3.1 Prenatal management
Patients with KTS are 10 times more likely to develop a DVT or embolism compared with the general population.9 In a retrospective investigation of 60 pregnant people with KTS in the Netherlands, 43% of patients experienced an aggravation of KTS-related symptoms during pregnancy. Furthermore, 5.8% of the patients developed a DVT, and 2.3% a pulmonary embolism. Compared with the standard population, these data indicate a highly elevated likelihood of developing a DVT or embolism, resulting from a vascular low-flow state combined with physiological prothrombotic changes in KTS patients.8
After consulting with the department of hemostaseology, we initiated an anticoagulation therapy with LMWH once daily to prevent thromboembolisms. Considering the patient's history of thromboembolisms, we increased the dose to twice daily in the last trimester. There was no registered thromboembolism throughout the course of the pregnancy.
In addition to the risk of life-threatening thromboembolisms during pregnancy, some studies reported a higher risk of intrauterine growth restriction.10, 11 Therefore, we performed regular ultrasound imaging throughout the pregnancy, which showed proper fetal growth.
3.2 Radiological imaging
Most case reports of pregnancy in KTS patients indicate that radiological imaging prior to the expected delivery date provides crucial information about the extent of the syndrome and any vascular malformations.8, 11-14 Consequently, we carried out a preoperative MRI. Although aggravation of KTS symptoms in pregnancy is well-documented, our patient's symptom level remained constant. However, we found a massive venous malformation intraoperatively (Figure 3). Neither was this finding identifiable in the preoperative MRI nor in any ultrasounds conducted during the pregnancy.
3.3 Mode of delivery
Due to the lack of significant scientific research, a general recommendation for the mode of delivery is not possible, and each pregnant patient with KTS must be discussed individually. According to earlier case reports, 44% of patients with KTS underwent C-sections. However, Horbach et al. found a significantly lower rate (8%) in their Dutch collective. This discrepancy was attributed to a “reporting bias” in the published case reports.8 The degree of the vascular malformations found in our patient was not covered in the study by Horbach et al., which lead us to the conclusion that KTS patients with reduced clinical features of the syndrome have a lower perinatal risk of bleeding or tissue damage. We also concluded that these patients may benefit from vaginal delivery due to the higher risk of bleeding and aggravated blood loss resulting from C-sections compared with vaginal delivery in the general population.
In this case, we decided, upon approval from the patient, to perform a C-section. After consulting with experts from the departments of radiology and angiology, we wanted to rule out the risk of aggravated or uncontrollable perinatal bleeding that could be caused by a rupture of the massive pelvic or vulvar vascular malformation during a vaginal delivery. The decision to perform a longitudinal laparotomy to reduce the risk of bleeding proved to be beneficial in retrospect considering the intraoperative finding of large parietal peritoneal varices, which were not distinguishable in the preoperative MRI and could have led to severe intraabdominal bleeding (Figure 3).
3.4 Postoperative period
Around 6 hours after the delivery, our patient showed a PPH under LMWH treatment. The bleeding stopped after we applied a pharmacological therapy, such as oxytocin and misoprostol.
Several studies indicate an elevated risk of PPH in KTS patients. However, Horbach et al. did not confirm any statistical significance compared with the reference population in the Netherlands. Furthermore, the role of LMWH in our patient's PPH remains unclear, as most KTS patients start LMWH treatment after delivery rather than during pregnancy.8
This is the first report of a postpartum deformation of the uterus in a KTS patient. We suggested further follow-up care and prompt medical intervention if the patient shows any symptoms or develops any reproductive disorders.
4 CONCLUSIONS
Due to the small number of case reports and limited scientific research about pregnancy in KTS patients, doctors have been discouraging KTS patients from getting pregnant up until today. Although there appears to be a high risk for complications, successful and uncomplicated pregnancies are possible for people with KTS. For this purpose, we believe a multidisciplinary strategy including prenatal consultations with radiology, anesthesia, hemostaseology, and pediatrics is crucial to minimize complications.
AUTHOR'S CONTRIBUTION
Konstantin Hofmann and Doris Macchiella developed the project, managed the data, and wrote the manuscript. Roman Kloeckner managed the data and wrote the manuscript. Annette Hasenburg wrote the manuscript.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
CONSENT
Written informed consent was obtained from the patient for the publication of this case and any accompanying images.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




