Association of autosomal-recessive-type distal renal tubular acidosis and Glanzmann thrombasthenia as a consequence of runs of homozygosity
Abstract
We report the case of a Filipino girl with autosomal-recessive-type distal renal tubular acidosis and Glanzmann thrombasthenia caused by homozygous variants in the genes SLC4A1 and ITGA2B within the long homozygous DNA region on chromosome 17q21.31. This haplotype may be retained among individuals of Filipino descent.
1 INTRODUCTION
Anion exchanger 1 (AE1), a chloride-bicarbonate exchanger, is encoded by the gene SLC4A1 located on chromosome 17q21.31 and is expressed at high levels on the basolateral membrane of alpha-intercalated cells of the collecting duct. Variants in SLC4A1 cause autosomal-recessive-type distal renal tubular acidosis (AR-dRTA), a rare genetic disorder characterized by metabolic acidosis, hypokalemia, hypercalciuria, and nephrocalcinosis.1, 2 Patients with AR-dRTA are limited in Southeast Asia, and AR-dRTA is often associated with hereditary spherocytosis or Southeast Asian ovalocytosis (SAO) because the AE1 protein is also expressed on the erythrocyte membrane.3 Moreover, ITGA2B, which encodes integrin alpha IIb subunit protein on the platelet surface is also located on chromosome 17q21.31 approximately 100 kb downstream of SLC4A1, and is responsible for Glanzmann thrombasthenia (GT). GT is a rare AR disorder characterized by platelet aggregation owing to insufficient binding of platelet surface glycoprotein and its ligand fibrinogen, resulting in severe hemorrhagic symptoms.4
Here, we report the case of a Filipino girl with AR-dRTA and GT wherein we performed molecular analysis. We identified homozygous variants in SLC4A1 and ITGA2B on chromosome 17q21.31 within the long homozygous DNA region, spanning more than 1200 kb.
2 CASE PRESENTATION
The proband was a 3-year-old girl who was the first child of Filipino parents without parental consanguinity. Her father and mother were from the Visayan Islands and Manila city, respectively, which are approximately 400 km apart. Her parents had mild bleeding tendency, and some relatives of her parents also had bleeding tendency and/or kidney disease (Figure S1); however, the actual disease status was unknown. The proband was admitted to Toho University Omori Medical Center because of frequent and intractable nasal bleeding at 10 months of age. GT was diagnosed owing to the lack of platelet surface glycoprotein alpha IIb, as demonstrated by flow cytometry. She required frequent transfusions of red blood cells and platelets because of intractable nasal or gingival bleeding, which occasionally resulted in hemorrhagic shock with a minimum Hb level of 2.4 g/dl. Thereafter, she developed platelet transfusion refractoriness owing to the production of anti-alpha IIb antibodies and required recombinant activated clotting factor VII to treat massive bleeding.
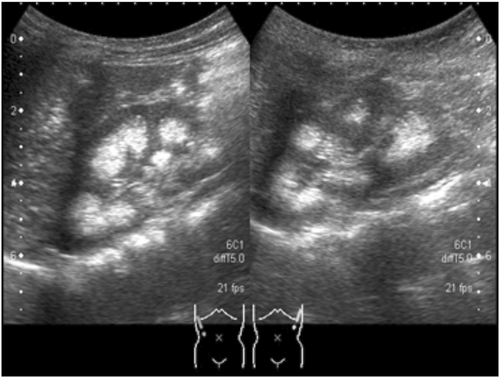
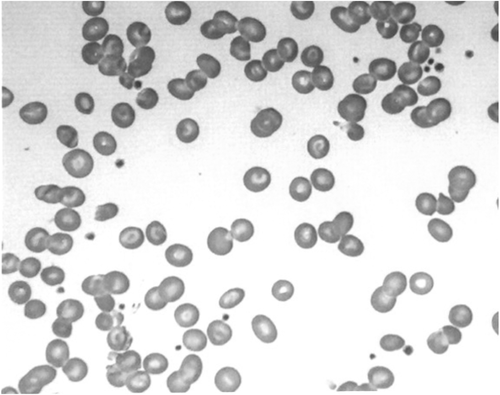
At 3 years of age, she exhibited repeated hypokalemia and metabolic acidosis during hemorrhagic episodes. Her height and body weight were 80.8 cm (−1.5 SD) and 8.9 kg (−1.7 SD), respectively (SD was calculated using auxological data of Filipino children5). Plasma potassium was 2.5 mEq/L, and blood gas analyses showed metabolic acidosis (pH: 7.276; HCO3: 10.5 mM; excess base: −14.6 mM; anion gap: 12.4 mM). Urine pH was inappropriately alkaline despite blood acidosis, and alkaluria was not ameliorated by administration of furosemide. We also observed hypercalciuria (urine Ca/creatinine ratio: 0.54) and bilateral nephrocalcinosis upon abdominal ultrasound (Figure 1) suggesting the diagnosis of dRTA. Bone radiography showed cupping and fraying of the bilateral metaphysis of the femur, indicating rickets (Figure 2). Although peripheral blood smears showed variable forms of erythrocyte deformities, such as elliptocytes, stomatocytes, and target cells (Figure 3), she did not experience apparent episodes of hemolysis, except for prolonged hyperbilirubinemia in early infancy.

Alkalinizing treatment using potassium sodium hydrogen citrate was initiated at 3 years of age. Her growth rate showed modest improvement because of poor drug adherence. However, the frequency of massive bleeding decreased after initiation of alkalinizing treatment; the number of hospitalizations due to massive bleeding was 2.6 times per year before 3 years of age (before initiation of alkalinizing treatment), 1.9 times per year between 3 and 7 years of age (due to bad drug adherence), and 0.6 times per year after 7 years of age (suggesting improvement of drug adherence), respectively.
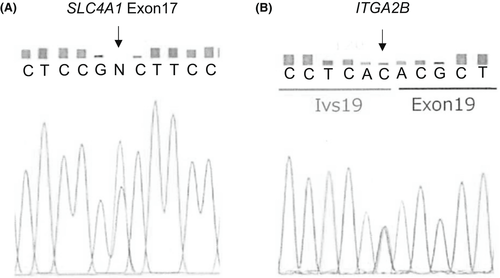
Next, we investigated whether AR-dRTA and GT occurred as a consensus genetic aberration because the genes responsible for these two disorders are located near each other on the same chromosomal site. Comprehensive genomic analysis using a TruSight One v1.0 sequencing panel (Illumina) was performed as described previously.6 Written informed consent was obtained from the patient's parents for genetic analyses. Homozygous single nucleotide variants within the splice donor site of ITGA1B (NM_000419.3: c.1946+1G>A, dbSNP ID: rs746091910) and within exon 17 of SLC4A1 (NM_000342.3: c.2102G>A, p.Gly701Asp, dbSNP ID: rs121912748) were identified. The homozygous region was expanded for more than 1200 kb in chromosome 17q21.31, which included the telomeric portions of the genes BRCA1, PYY, NAGS, G6PC3, SLC4A1, and ITGA2B. To elucidate the underlying genetic mechanisms of the homozygous variants, we performed Sanger sequencing for SLC4A1 and ITGA2B and found that both parents were heterozygous for the same variants (Figure 4), indicating that identical haplotypes were inherited from each parent as a result of linkage disequilibrium. Moreover, the long homozygous DNA region on chromosome 17q21.31 including SLC4A1 and ITGA2B indicated that association of dRTA and GT was an example of deleterious homozygotes in runs of homozygosity.7
3 DISCUSSION
To the best of our knowledge, this is the first case report of a molecularly confirmed association between dRTA and GT. Anacleto et al.8 also reported the case of two Filipino siblings with dRTA accompanied by GT; however, the genetic basis of GT was not analyzed. Interestingly, these siblings were domiciled in the Visayan Islands and had the same variant in SLC4A1 as in our case. The authors speculated that an association of dRTA and GT might be by chance because the association of these two diseases was not reported previously and because SLC4A1 and ITGA2B are closely situated on the same chromosomal site, and it is very unlikely that they will be separated by crossing over at meiosis. However, we identified homozygous variants in SLC4A1 and ITGA2B on chromosome 17q21.31 in our case. Moreover, the long homozygous DNA region, including SLC4A1 and ITGA2B, was expanding in chromosome 17q21.31. Pemberton et al.9 reported that intermediate runs of homozygosity (hundreds of kb to 2000 kb) could result from background relatedness, and that long runs of homozygosity (over 1000–2000 kb) could arise from recent parental relatedness. The long homozygous DNA region, expanded for more than 1200 kb, may have originated from a common ancestor, although the patient's parents denied consanguinity and were from districts approximately 400 km apart. Therefore, this disease-associated haplotype may be retained among individuals of Filipino descent, and hence, the frequency of patients with AR-dRTA accompanied by GT requires future study. In that case, chromosome microarray is considered useful to investigate runs of homozygosity and degree of relatedness.
Interestingly, the frequency of massive nasal or gingival bleeding decreased after the initiation of alkalinizing treatment. One explanation for this observation is that acidosis itself may impede the hemostatic functions of platelets and clotting process. Etulain et al.10 reported that extracellular acidosis downregulated platelet responses, such as adhesion, shape change, aggregation, and granule secretion, in an ex vivo assay. Acidosis also compromises the clotting process by accelerating fibrinogen consumption.11 Similar mechanisms may have contributed to massive and frequent hemorrhagic episodes in our case. Alkalinizing treatment might be effective in decreasing hemorrhagic episodes in patients with AR-dRTA accompanied by GT.
AR-dRTA and GT are serious by themselves; however, association of the two diseases could be life-threatening. If physicians observe patients with AR-dRTA or GT in Southeast Asia, especially in the Philippines, the possibility of the association of the two diseases should be considered.
4 CONCLUSION
We report the case of a Filipino girl who developed AR-dRTA and GT because of homozygous variants in the genes SLC4A1 and ITGA2B on chromosome 17q21.31. This disease-associated haplotype may be retained among individuals of Filipino descent because the long homozygous DNA region including these genes was expanded in chromosome 17q21.31. Therefore, the frequency of patients with AR-dRTA accompanied by GT needs to be determined in a future study.
AUTHOR CONTRIBUTIONS
Keiko Aso collected and analyzed the data and drafted and revised the manuscript. Takehiko Soutome collected and analyzed the data. Mari Satoh reviewed and revised the manuscript. Takako Aoki carried out the genomic analyses. Hiromi Ogura carried out the genomic analyses. Toshiyuki Yamamoto carried out the genomic analyses, contributed to clinicogenetical diagnosis, and reviewed the manuscript. Hitoshi Kanno carried out the genomic analyses, contributed to clinicogenetical diagnosis, and reviewed the manuscript. Hiroyuki Takahashi reviewed and revised the manuscript.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Faculty of Medicine, Toho University, Tokyo, Japan (approval no. A19054).
CONSENT
The parents of the patient provided written informed consent for genetic analysis and to publish this case report, including case description, medical data, and images.




