Immediate postnatal central hypothyroidism caused by maternal Graves' disease: Importance of early screening
Abstract
This report illustrates a case of central hypothyroidism in a newborn immediately after birth caused by maternal Graves' disease. Infants from mothers with Graves' disease require careful examination without waiting for neonatal screening results, even though the mother's thyroid function is normal at birth or the newborn does not have goiter.
1 INTRODUCTION
Graves' disease (GD) during pregnancy has varying effects on fetal/neonatal thyroid function. Fetal/neonatal thyroid function can be disturbed with a high amount of variation in the type of effects and severity. These effects and severity are dependent on the presence of antithyroid antibodies, the use of antithyroid drug (ATD) and/or potassium iodide (KI) treatment, and the maternal thyroid hormone state.1, 2 In particular, excess maternal iodine ingestion during pregnancy might cause fetal/neonatal hypothyroidism and goiter.3 Moreover, in infants born to mothers with inadequately treated GD, central hypothyroidism is one of the key complications, but its risk may be underestimated.2, 4 A neonatal screening program with testing of simultaneous thyroid-stimulating hormone (thyrotropin; TSH) and thyroxine (T4) may be able to identify these newborns born to mothers with GD, but this may not be sufficient to improve the prognosis of the child.
We report here an infantile case of central hypothyroidism with extremely low free thyroxine (FT4) levels without goiter immediately after birth that were caused by maternal GD. This report highlights the importance of testing thyroid function without waiting for neonatal screening results in newborns born to mothers with inadequately treated GD.
2 CASE PRESENTATION
A 27-year-old woman was referred to our hospital because of gestational hypertension at 31 weeks gestational age (wGA). She was diagnosed with GD previously. However, when she visited our hospital, she had not been treated for GD because of her poor compliance with medication. Her serum TSH concentration was <0.02 μIU/mL (adult normal value: 0.541–4.261 μIU/mL), free triiodothyronine (FT3) concentration was 9.35 pg/mL (adult normal value: 2.39–4.06 pg/mL), FT4 concentration was 3.06 ng/dL (adult normal value: 0.76–1.65 ng/dL), and thyrotropin receptor antibody (TRAb) concentration was 5.4 IU/L (third-generation assay, adult normal value: <2.0 IU/L). At 32 wGA, she started receiving methimazole treatment (10 mg daily). At 35 wGA, despite methimazole treatment, her serum TSH concentration was <0.02 μIU/mL and FT4 concentration was 2.59 ng/dL. Therefore, she started receiving oral KI treatment (0.71 mg/kg daily) concurrently. Four days before childbirth, her FT3 and FT4 concentrations returned to normal (TSH, <0.02 μIU/mL; FT3, 3.75 pg/mL; FT4, 1.41 ng/dL). After receiving methimazole treatment for 32 days and KI treatment for 10 days before delivery, she delivered by cesarean section at 37 wGA.
The female newborn weighed 2534 g and had no abnormal signs. Ultrasonography showed that the thyroid gland was eutopic, had a normal size, and had no nodules. The epiphysis of the distal end of the femur was detectable on radiography. The infant had normal pituitary magnetic resonance imaging findings. The total iodine in urine on the day of birth was high (2591 μg/g·creatinine). In cord blood, the TRAb concentration was 4.4 IU/L and TSH-stimulation blocking antibody (TSBAb) was 0.0%. The thyroid hormone status of the infant and levothyroxine (LT4) doses over time are shown in Figure 1. Laboratory thyroid tests of the infant's serum immediately after birth were as follows: TSH concentration, 0.21 μIU/mL; FT3 concentration, 1.53 pg/mL; and FT4 concentration, 0.57 ng/dL. A neonatal TSH surge was not observed, and FT3 and FT4 concentrations were low. One day after birth, a lower FT4 concentration was also observed (0.50 ng/dL), and LT4 treatment of 10 μg/kg/day was initiated. The LT4 dose was then adjusted to obtain FT4 concentrations within the age-adjusted normal range. At 37 days old, the infant's TRAb concentration was not detected for the first time. An increase in the TSH concentration was first observed at 109 days old under the treatment of LT4 4.5 μg/kg/day. At follow-up at 1 year of age, the infant had normal growth and neurodevelopment.
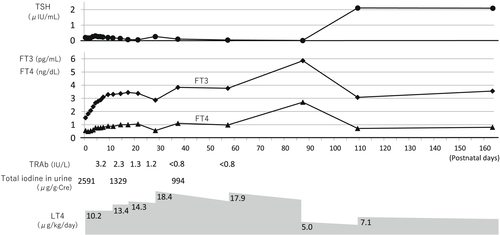
3 DISCUSSION
GD during pregnancy has varying effects on fetal/neonatal thyroid function. Maternal thyroid hormone, thyroid-stimulating antibody (TSAb), ATDs, and KI pass through the placenta and affect fetal/neonatal thyroid function. In infants born to mothers with GD, maternal TSAb-related primary hyperthyroidism, otherwise known as “neonatal GD”, can develop. Abeillon-du Payrat et al.5 reported that maternal TRAb (second-generation assay) values >5 IU/L in the second trimester of pregnancy indicate a risk of neonatal hyperthyroidism. Neonatal GD is temporary and lasts a few weeks to a few months until the mother's antibodies disappear. In infants born to mothers with GD, gestational GD-related central hypothyroidism (gGD-CH) can also develop. This condition is due to the transfer of maternal thyroid hormone and TSAb through the placenta and fetal/neonatal TSH suppression, which leads to a blunted ability to establish the negative feedback system of the pituitary–thyroid axis. Fetal thyroid hormone and TSH secretion start to increase at approximately 12 wGA, and TSH regulation by T4 is established at approximately 24–27 wGA.1 Nakano et al.4 reported that fetal TSH suppression for 5–7 weeks after 22 wGA could lead to the development of gGD-CH in infants born to mothers with uncontrolled gestational GD during pregnancy. gGD-CH is also temporary and lasts a relatively longer period compared with neonatal GD. In our case, we speculate that the infant did not have elevated thyroid hormone concentrations because maternal thyroid hormone and TSAb concentrations late in pregnancy were not high. In contrast, we speculate that gGD-CH was relatively severe, and at least 3 months were required to recover the autocrine ability of TSH. Collectively, in infants born to mothers with GD, maternal thyroid hormone and “neonatal GD” contribute to fetal/neonatal hyperthyroidism, but maternal ATDs and/or KI and gGD-CH contribute to fetal/neonatal hypothyroidism.
Oral KI treatment during pregnancy might have some adverse effects on the fetus. The thyroid gland reduces thyroid hormone production in the presence of excess iodine (Wolff–Chaikoff effect). This effect is temporary, and, within a few days, thyroid hormone synthesis returns to normal through the so-called “escape phenomenon”. In the fetal period, the Wolff–Chaikoff effect matures towards the end of gestation.6 Because the escape phenomenon occurs less often in fetuses than in mothers, high-dose maternal KI treatment in late pregnancy may be at risk of strongly inducing neonatal primary hypothyroidism. In our case, we speculate that one of the causes of the infant's extremely low FT4 concentrations immediately after birth was maternal KI treatment after mid-pregnancy.
Fetal goiter associated with maternal GD can result from either fetal hyperthyroidism or hypothyroidism. In fact, fetal goiter is found in 19% of fetuses carried by mothers with GD.7 The fetal pituitary secretes TSH after 12 wGA, and fetal TSH receptors become responsive to TSH after 20 wGA.1, 8 Maternal TSAb crosses the placenta and can overstimulate the fetal thyroid, causing hyperthyroidism and goiter.8 When the mother receives ATD and/or KI treatment after mid-pregnancy, the fetal thyroid sometimes becomes enlarged. This enlargement is likely caused by the hyperstimulation of tissue due to increased levels of fetal TSH concentrations in response to fetal thyroid insufficiency.3, 8, 9 Because fetal goiter is an important sign of fetal hyperthyroidism and hypothyroidism, fetal thyroid ultrasonography for mothers with GD is usually carefully performed. However, as in our case, in a fetus with gGD-CH caused by poor treatment of the mother with GD until mid-pregnancy, fetal goiter may not occur because of decreased fetal TSH secretion.
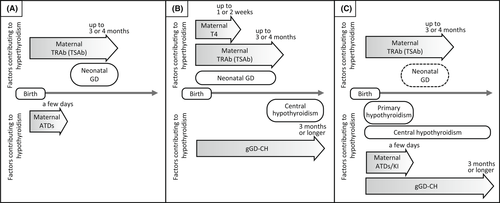
Figure 2 shows three different situations of infants born to mothers with GD. (i) In infants born to mothers in whom GD is well controlled by ATD treatment, neonatal GD can develop after the disappearance of maternal ATDs within days (Figure 2A). (ii) In infants born to mothers with poorly controlled GD, there is a high risk of developing neonatal GD shortly after birth and then developing gGD-CH (Figure 2B). (iii) As in our case, in infants born to mothers who are untreated until mid-pregnancy and receive ATD and/or KI treatment in late pregnancy, hypothyroidism might have been present for a relatively long time from immediately after birth. This hypothyroidism is due to the effects of gGD-CH. Additionally, the effects of maternal ATDs and/or KI may be the cause of neonatal primary hypothyroidism in the first few days of life. Moreover, if the mother's TRAb (TSAb) value is high, her infant may show “triphasic changes” through primary hypothyroidism, neonatal GD, and gGD-CH (Figure 2C). When treating infants born to mothers with GD, the infant's treatment needs to be considered by taking into account the course of the mother's thyroid function and treatment during pregnancy.
Neonatal screening programs are an invaluable public health tool. Neonatal screening based on TSH determination allows the diagnosis of primary congenital hypothyroidism (CH) but misses central CH. Adding T4 to TSH measurements enables the identification of central CH as a prevalent condition, and contributes to recognizing other thyroidal disorders and congenital multiple pituitary hormone deficiency.10 Additionally, a T4-based neonatal CH screening program appears useful in detecting gGD-CH. Kempers et al.2 reported the case histories of 18 infants with central CH who were born to 17 mothers with untreated or inadequately treated GD in the Netherlands where a T4-based neonatal CH screening program had been introduced. More than half of these infants were already hypothyroid at the first thyroid function measurements, although six became hypothyroid after an initial euthyroxinemic phase and one became hypothyroid after a short hyperthyroid phase. Moreover, all women with GD who gave birth to children with central CH were inadequately treated. In general, neonatal screening samples are collected between 48 hours and 7 days after birth to avoid the effect of TSH surge soon after birth. Our case is one of the few cases of neonatal gGD-CH with extremely low FT4 concentrations, which were confirmed and treated immediately after birth (before neonatal screening testing). The patient's extremely low FT4 concentrations immediately after birth may have been caused not only by gGD-CH but also by maternal ATD and KI treatment after mid-pregnancy. Our findings suggest that, in cases where a mother is inadequately treated GD prior to mid-pregnancy, even though the mother's thyroid function is normal at birth, the newborn may have severe hypothyroidism immediately after birth.
In conclusion, in newborns born to mothers with inadequately treated GD, testing thyroid function without waiting for neonatal screening results should be considered. This recommendation is based on the assumption that FT4 concentrations are low immediately after birth, even though the mother's thyroid function is normal at birth or the newborn does not have goiter.
AUTHOR CONTRIBUTIONS
Saho Tochibora wrote the manuscript. Saho Tochibora, Tomohiro Hori, Mai Mori, Hideki Matsumoto, Hiroki Otsuka, Hideo Sasai, Yuko Ito, and Yukiko Kasahara managed the patient and performed critical revision of the manuscript. Norio Kawamoto and Hidenori Ohnishi supervised the treatment of the patient and critically reviewed the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank the patient's parents for the permission to report the clinical course of the patient's condition.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient's parents for the publication of this case report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




