Co-infection of Klebsiella pneumonia, Cytomegalovirus, Aspergillus and Zygomycete in a patient with SARS-CoV-2
Abstract
Co-infection between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other pathogens has become a serious threat. There are the reports of fungal, bacterial, and viral co-infections with SARS-CoV-2. We report the unusual case of concomitant aspergillosis, mucormycosis, cytomegalovirus pneumonia, and also klebsiella pneumoniae empyema as the complication of SARS-CoV-2.
1 INTRODUCTION
Secondary infections or co-infections are commonly identified in the severe influenza and other severe respiratory viral infections with high mortality and morbidity rates.1 Co-infection between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other pathogens has become another serious threat to treat the patients, which should not be neglected.2 These co-pathogens include bacteria such as Streptococcus pneumoniae and Staphylococcus aureus, fungal pathogens including Aspergillus or Mucorales species, and the viruses such as influenza, and rhinovirus/enterovirus.3 Hence, we report the first case of concomitant aspergillosis, mucormycosis, cytomegalovirus (CMV) pneumonia, and klebsiella pneumoniae empyema as the complication of COVID-19 infection who had a favorable clinical outcome.
2 CASE PRESENTATION
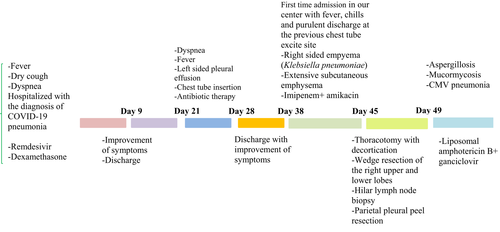
A 68-year-old diabetic man was presented to the emergency department with a 5-day history of fever (38.7°C), dry cough, and dyspnea (SpO2 was 90%—room air). He was on the oral hypoglycemic agent (metformin 500 mg every 12 h) for several years. Laboratory studies showed elevated C-reactive protein (120 mg/dl), and reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 diagnosis. A high-resolution chest CT scan obtained at admission was consistent with COVID-19 pneumonia. The patient received remdesivir and dexamethasone and was discharged after 9 days with the improvement of signs and symptoms. After 12 days, the patient was re-admitted because of severe dyspnea and fever. Chest CT scan revealed left-sided pleural effusion, and the chest tube was inserted and discharged after 7 days with the improvement of dyspnea. After 10 days, the patient was referred to our center for the first time because of malaise, fever, chills, and purulent discharge at the previous chest tube excite site.
At admission, the patient was febrile, and the vital signs were as follows: PR: 110 beats/min, RR: 25 breath/min, T: 38.5, and BP: 120/90 mmHg. The initial laboratory data were as follows: white blood cell count (WBC): 4900/mm3 (neutrophils: 83%, lymphocytes: 8%), hemoglobin (Hb): 10.2 g/dl, platelets (PLT): 250,000/mm3, blood sugar: 187, and creatinine: 1.1 mg/dl. Furthermore, COVID-19 PCR was reported positive. According to pleural effusion observed in chest CT scan, thoracentesis was performed and pleural fluid was sent for analysis, bacterial and fungal smear, and culture. Blood cultures were negative. The pleural fluid analysis was as follows: turbid appearance, white blood cell >500,000, polymorphonuclear 90%, lactate dehydrogenase (LDH) 26,448 U/L, sugar <10 mg/dl, protein 2.9 g/dl, and albumin 1.9 g/dl. Serum LDH level, total protein, and albumin were 523 U/L, 5.1 g/dl, and 3.2 g/dl, respectively, indicative of empyema, and a chest tube was inserted.
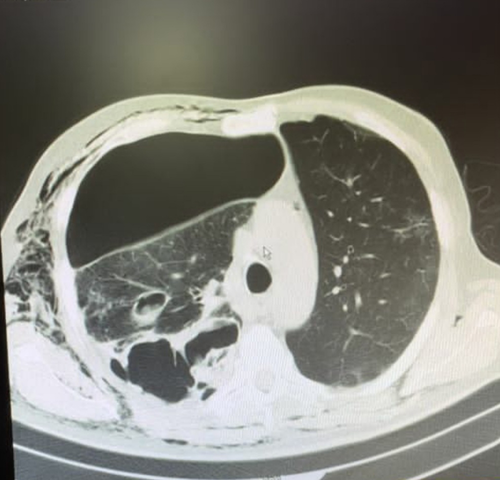
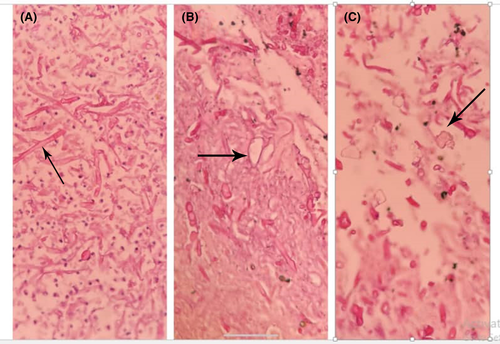
Imipenem 500 mg every 6 h and vancomycin 1 g every 12 h were started as empiric treatment. The result of culture was reported klebsiella pneumoniae, which was sensitive to meropenem and imipenem. The vancomycin was discontinued, and imipenem with the addition of amikacin was continued. The patient presented extensive subcutaneous emphysema on both sides, and a left chest tube was inserted (Figure 1). After several days, the left-sided chest tube came out. According to the right-sided empyema and no significant improvement, the patient underwent right thoracotomy with decortication. Besides, wedge resection of the right upper and lower lobes, hilar lymph node biopsy, and parietal pleural peel resection were done. The patient's whole lung was extended after the procedure, and an anterior and posterior chest tube was placed. In the histopathology, right upper lobe wedge resection revealed a severe suppurative necroinflammatory process associated with the presence of a few partially degenerated fungal hyphae (both wide and irregular). Right lower lobe wedge resection showed extensive severe suppurative necroinflammatory process associated with the presence of numerous fungal hyphae (both wide and irregular). Moreover, the vessel wall infiltrated by several fungal hyphae, including thin and broad fungal hyphae and calcium oxalate crystals accompanied by fungal hyphae, was the indicative of aspergillosis (Figure 2).
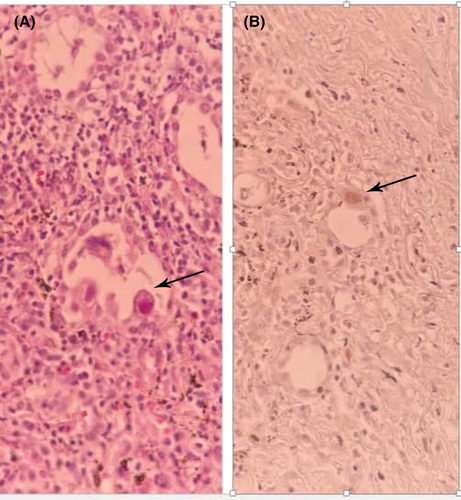
Hilar lymph node biopsy revealed a fibro-inflammatory process including foci of anthracotic pigment deposition. Fibro-adipose tissue showed bland-looking gland-like structures, patchy lymphocytic infiltration, and the presence of a few large cells with cytopathology suggestive of CMV infection. Immunohistochemistry (IHC) was performed and confirmed the diagnosis of CMV pneumonia (Figure 3).
According to the invasive fungal infection with both Aspergillus and Mucoraceae species and with the diagnosis of CMV disease, the liposomal amphotericin B, 5 mg/kg/day, and intravenous ganciclovir 5 mg/kg every 12 h were initiated. Furthermore, the imipenem with amikacin was continued for Klebsiella pneumoniae empyema. The patient signs and symptoms were improved and were afebrile. After the discontinuation of liposomal amphotericin B and intravenous ganciclovir course, posaconazole oral suspension 200 mg every 6 h and valganciclovir 900 mg every 12 h were started. The patient was discharged after one month with a favorable clinical outcome (Figure 4).
3 DISCUSSION
The possibility of SARS-CoV-2 co-infections with bacterial, fungal, and viral pathogens should not be neglected. Fungal co-infections among hospitalized patients with coronavirus disease 2019 (COVID-19) are a challenging issue. Few recent research have shown instances of COVID-19 infection involving more than one aspergillus species or two separate fungal infections at the same time. Toc et al. reported a case of COVID-19-associated pulmonary aspergillosis with Aspergillus fumigatus and Aspergillus flavus with favorable outcome.2 Johnson et al.3 reported a case of combined probable pulmonary aspergillosis and possible mucormycosis in a diabetic male with COVID-19 in the ICU who was treated with liposomal amphotericin B.
Critical illness is an important risk factor for CMV reactivation in terms of the immunosuppression. Although co-infections with CMV are frequent in critically ill patients, but the impact on COVID-19 patients is unclear. Moniz et al.4 presented five case reports of CMV reactivation in COVID-19 patients admitted to the ICU with respiratory failure. It was well described that critical or severe illness can induce immune suppression. The most common laboratory finding in patients with COVID-19 is lymphopenia, which correlates to disease severity. The loss in cell-mediated immunity, particularly the drop in T-cell numbers, has recently been discovered to be the most important factor in disease severity.5 On the contrary, the number of iatrogenic factors, including the usage of corticosteroids in the treatment of severe COVID-19, may predispose the patients to other infections.6 In addition to ICU admission and mechanical ventilation, other entities, including transfusions, sepsis, corticosteroids, and acute respiratory distress syndrome, were found to be associated with the risk of CMV reactivation.7
Co-infections with bacteria and fungi may exacerbate the course of COVID-19 infection in a small percentage of individuals. In a retrospective study, the second most common respiratory pathogen detected from patients with COVID-19 was Klebsiella pneumoniae.8, 9 Montrucchio et al.10 reported patients with COVID-19-related acute respiratory distress syndrome who developed invasive infections in terms of carbapenemase-producing Klebsiella pneumoniae. In our patient, the culture of empyema and visceral pleural peel confirmed the superimposed of Klebsiella pneumoniae infection on COVID-19.
Tissue invasion by a filamentous fungus via the histopathological examination of biopsy provides a diagnosis of invasive fungal infections, and positive culture of Aspergillus species from the specimen is required to make a proven diagnosis of invasive aspergillosis.11 In our case, right upper lobe wedge resection and right lower lobe wedge resection revealed wide and irregular fungal hyphae indicative of mucormycosis. Moreover, the vessel wall infiltrated by several thin and broad fungal hyphae. Calcium oxalate crystals accompanied by these fungal hyphae were indicative of aspergillosis. Definitive diagnosis of CMV pneumonia is determined based on the lung tissue samples by histopathologic testing and immunohistochemical analysis.12 Our case was consistent with the proven CMV pneumonia. According to the histopathology and IHC, our patient had pulmonary aspergillosis, mucormycosis, and CMV disease with Klebsiella pneumoniae infection. The combined risk factors, including diabetes mellitus, recent corticosteroid treatment, and COVID-19 per se, contributed to concomitant infections in our patient, which is a unique report.
4 CONCLUSION
Secondary infections or co-infections are identified between SARS-CoV-2 and other pathogens. This entity has become another serious threat that should not be neglected.
AUTHOR CONTRIBUTIONS
P.T, M.PT, A.H, Z.A, A.M, M.R, and M.MD acquired, analyzed, and interpreted the data. A.H wrote the first draft of the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
None.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
ETHICAL APPROVAL
This research was approved by the ethics committee of Shahid Beheshti University of Medical Sciences, and written informed consent was obtained from the patient.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this case report type article as no new data were created or analyzed in this study.




