Alpha-L-iduronidase deficiency: A novel mutation resulting in severe early presentation of Mucopolysaccharidosis type I and literature review of the molecular basis
Funding information
There is no funding to report for this submission
Abstract
The IDUA gene (MIM 252800) provides instructions for producing alpha-L-iduronidase, which is essential for the breakdown of large sugar molecules called glycosaminoglycans (GAGs). Mutations in the IDUA gene have been found to cause Mucopolysaccharidosis type I (MPS I) (MIM 607014). This leads to the accumulation of GAGs within lysosomes causing many different organs and tissues to be dysfunctional. Deleted IDUA gene has not been reported in the literature, which showed to be associated with a severe phenotype in our proband case. We report a child from a consanguineous family who presented with severe cardiogenic shock attributed to dilated cardiomyopathy. He was also found to have hepatosplenomegaly, joint stiffness, hearing loss, corneal hazing, facial dysmorphism, and dilation of brain ventricles. Lysosomal storage disease particularly MPS I was suspected though it is considered to be an early atypical presentation. The diagnosis was achieved via gene mutation analysis which showed homozygous IDUA deletion of exon 9′ to 3′ in combination with a severe deficiency of alpha-L-iduronidase enzyme. A variant in the form of IDUA gene deletion may indicate an early severe phenotypic presentation of MPS I. Establishment of the diagnosis permits genetic counseling, prevents patients from undergoing unhelpful diagnostic procedures, and allows for accurate prognosis.
1 INTRODUCTION
Mucopolysaccharidosis type 1 (MPS 1) is one of the rare Lysosomal storage disorders inherited in an autosomal recessive manner. A deficiency of α-L-iduronidase enzyme is the reason for the pathological accumulation of heparan sulfate and dermatan sulfate in various tissues of the body. The pathological variants of the IDUA gene are responsible for the deficiency of α-L-iduronidase enzyme. Those variants have been defined as nonsense, missense, splice site, and minor deletions and/or insertions. Of note, there is no reported exon or whole-IDUA deletions or duplications.1
MPS 1is a progressive multisystem disorder with a wide spectrum of clinical variability ranging from life-threatening to mild clinical manifestations. Historically, the disease is classified into three major subtypes based on the rapidity of progression and the degree of cognitive involvement which makes predicting patient phenotype important for genetic counseling and the decision regarding therapy challenging. However, IDUA gene, variant analysis can build up knowledge regarding the prediction of the clinical range of MPS-I phenotypes.1, 2
Hurler syndrome is defined as the most severe form of MPS type I, with the earliest diagnosis and treatment initiation. The onset of symptoms is approximately 6 months. The more attenuated forms Hurler–Scheie and Scheie syndromes are characterized by symptoms experienced beyond infancy.2
2 CASE SCENARIO
A three-month-old Iranian male infant presented to the emergency department with a one-month history of shortness of breath, grunting, and sweating during feeds. The shortness of breath was progressive. A few days before admission, the mother noticed that the infant was not able to feed and was less active. The family denied a history of fever, cough, or contact with sick patients.
The general appearance of the patient showed that he had frontal bossing and subtle coarse facial features. He was in severe respiratory distress with signs of shock. the patient had a depressed anterior fontanelle, poor peripheral pulses, with tachycardia (>150 BPM). He was grunting with the use of accessory muscles. He also had a deformed chest in the form of a bulging sternum and Harrison sulcus. Abdominal examination showed firm hepatosplenomegaly and bilateral inguinal hernias. There were multiple Mongolian spots on his back.
Family history revealed that the patient was the third child of healthy parents with a consanguineous relationship. There was no family history of similar symptoms. His mother had a fetus that was diagnosed antenatally with gastroschisis and subsequently aborted on the 5th month of pregnancy. No further details were provided on the nature of the diagnosis.
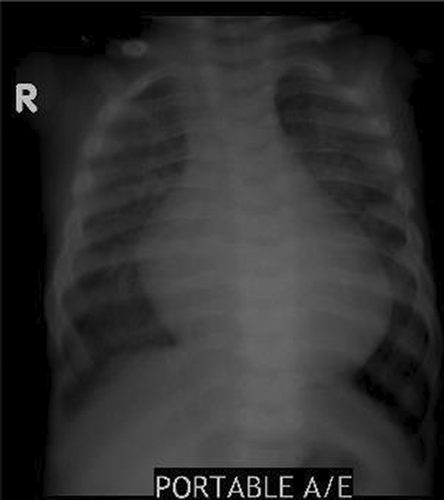
The investigations revealed cardiomegaly and congested lung on chest radiograph Figure 1.
Echocardiography showed a dilated left atrium and left ventricle, with left ventricular hypokinesia. There was a prolapsing mitral valve resulting in moderate mitral regurgitation, with severe left ventricular dysfunction. The Ejection Fraction was significantly affected with a value of 20%–22%. The patient was thus admitted to the Pediatric intensive care unit (PICU) for about 2 weeks with a diagnosis of cardiogenic shock. He required ventilator nasal support and inotropes.
The patient was referred for an ophthalmological examination clinic for a consultation which confirmed haziness in the sub-corneal layer suggestive of corneal clouding.
Based on multisystem involvement, a lysosomal defect etiology was suspected, so further metabolic and genetic investigations were performed in parallel. The lysosomal enzyme levels showed a significantly low level of Alpha-1-iduronidase enzyme while the whole-exome sequencing revealed normal IDUA sequencing, so MLPA study of IDUA gene was performed which showed significant deletion of multiple exons (Table 1).
| Test | Results | Interpretation |
|---|---|---|
| Lysosomal enzyme assay | 0.0 mcmol/h (>1.5) | Low |
| Whole exome sequencing | No pathogenic variant was detected | Normal |
| IDUA MLPA | Deletion of exon 9′–3′ | Novel variant, Likely pathogenic |
Establishing the diagnosis of MPS type I required approximately 12 weeks as a validated molecular test time frame is about few weeks to months. During follow-up period, the infant experienced musculoskeletal manifestations in the form of restricted joint movement affecting multiple joints symmetrically, and a gibbous lumbar deformity which highlights the severity and rapidity of disease progression.
A bone marrow transplantation (BMT) was performed at the age of 1 year. The patient received cells from one of his relatives who matched one haplotype. This procedure was complicated by the development of graft-versus-host disease, with the regression of organomegaly, cardiomyopathy, and hypotonia.
3 DISCUSSION
Mucopolysaccharidosis type I (MPS-I) is a lysosomal inherited disease resulting from a pathogenic variant in the α-L-iduronidase (IDUA) gene. These variants lead to a defect of the glycosidase α-L-iduronidase (IDUA), which plays a fundamental role in the degradation of heparan sulfate and dermatan sulfate. The pathogenic accumulation of those glycosaminoglycan in variable body tissues results in variable MPS type1 clinical features. The clinical phenotypes in MPS-I range over a continuum spectrum of severity (Hurler syndrome, severe; Hurler/Scheie syndrome, intermediate; Scheie syndrome, mild). The severity and disease progression is difficult to predict as there are no easily measurable biochemical differences. However, a large proportion of pathogenic variants are well known and recurrent, thus may be beneficial in predicting phenotype.1, 3
IDUA gene contains 14 exons and spans approximately 19 kp. The first 2 exons are separated by an intron of 566 bp; followed by a large intron of approximately 13 kp, and the last 12 exons are clustered within 4.5 kb.4
The intron 2 is characterized by the presence of an Alu repetitive sequence and a highly polymorphic variable number of tandem repeats which attributes to benign variant.4
A total of more than 110 pathogenic variants have been identified as causative of MPS-I consisting of nonsense, missense, splice site, and minor deletions and/or insertions. Moreover, polymorphisms or nonpathogenic sequence variants have been identified.4, 5
More intragenic variants that may lead to severe phenotypes are expected to be discovered in the future; while it is expected to have a limited number of pathogenic variants that may result in attenuated MPS I.
IDUA has an overlapping transcript functioning to code for a putative sulfate transporter termed Sat-1 (SLC26A6). There are no reported pathogenic variants affecting SLC26A6 in the literature, however, it is believed that pathogenic variants may negatively affect the function of both IDUA and SLC26A6 resulting in a complex phenotype. 4, 5
All patients with identified homozygous nonsense variants experience a severe phenotypic form of MPS I, while the phenotypes associated with insertion, deletion, missense, or splice site pathogenic variants are unpredictable and much more variable. Some residual enzyme activity is usually observed in missense pathogenic variants particularly, the R89Q which has been observed in several mild phenotypes even when it is found in combination with a nonsense mutation.4, 5
Homozygosity or compound heterozygosity of the common p. Gln70Ter or p. Trp402Ter pathogenic variants, permit a complete loss of IDUA enzyme activity and are associated with severe MPS I. Any combination of two "severe" variants leads to a severe form of MPS I.5
A combination of one "severe" variant and a second pathogenic variant allows some activity of the enzyme; however, there are many identifiable non-recurrent pathogenic variants, which identify an emerging complex picture of molecular heterogeneity. This adds challenge to accurately predicting phenotype-genotype correlation.5
The delay in diagnosis of this rare lysosomal storage disorder is not uncommon given the rarity of the disease and the complex heterogeneous phenotype. In the index case, there was no significant delay in the diagnosis as the patient phenotype was severe and early. This finding highlights that a deletion of exon 9′–3′ affecting IUDA predicts an early-onset severe phenotypic form.5
4 CONCLUSION
Mucopolysaccharidosis type I is a rare lysosomal storage disease that has a continuum of clinical features ranging from severe life-threatening features to mild attenuated phenotype. This has been attributed to the genetic heterogeneity of the IDUA gene. Single exome or multiple exome deletion has not been reported yet in the literature, thus its clinical impact is not known. The clinical features of the index case suggest that intragenic exomes deletion is responsible for a severe phenotype of MPS type I.
AUTHOR CONTRIBUTIONS
N. Al Zaabi the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
ACKNOWLEDGMENTS
We are grateful to our patients and their families for their contributions to this work.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Signed informed consent was obtained from the patient's parents in accordance with the Ministry of health and prevention Research Ethics Committee (REC). This study was exempted from Ethical approval.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.