Free-wall perforation of the left ventricle after acute myocardial infarction complicated with pericardial tamponade that mimicked ascending aortic dissection: A case report
Funding information
None
Abstract
Life-threatening cardiac events may be misdiagnosed as acute aortic dissection because of notable symptom mimicry. We report the case of a 72-year-old male patient with presentations presumed to be of aortic origin. However, surgery revealed posterior free-wall perforation in the left ventricle caused by the occlusion of an obtuse marginal branch.
1 INTRODUCTION
Acute aortic dissection (AAD) is the leading cause of mortality from aortic pathology. However, AAD is often misdiagnosed because of notable presentation overlap with other life-threatening cardiac events. For example, symptoms of AAD can mimic those of acute coronary syndrome with potentially catastrophic consequences.1-4 There are reports of AAD complicated by acute myocardial infarction (AMI) with coronary artery malperfusion for which an incorrect diagnosis and consequent mistreatment may prove fatal.1, 3 There have been no reports describing free-wall rupture mistaken for AAD. We describe the case of a 72-year-old male patient who presented with severe chest pain, abnormalities surrounding the aorta (revealed by CT), and hemodynamic instability; all the symptoms were preliminarily presumed to be AAD related.
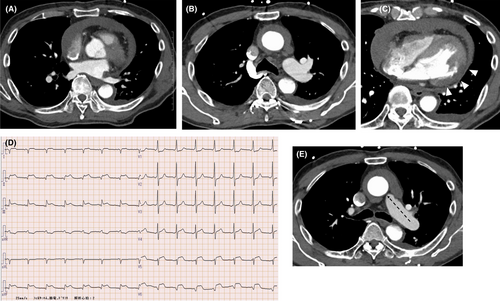
Computed tomography (CT) is a rapid and non-invasive diagnostic tool with a documented ability for detecting aortic disease.5 For patients with suspected type A aortic dissection, a CT scan is the recommended first line of investigation. However, imaging quality is affected by the heartbeat3 and CT visualization of the aortic root or ascending aorta may not suffice for conclusive AAD diagnosis. The preliminary chest CT of our patient revealed a crescent-shaped filling defect surrounding the ascending aorta with characteristics of intimal hemorrhage, hypoperfusion in the lateral to posterior wall of the left ventricle (LV), and cardiac tamponade and inferior wall ST elevation was identified on the electrocardiogram (ECG); all the findings supported the diagnosis of potential AAD with coronary malperfusion. However, because the complication of cardiac tamponade was significant, accurate diagnosis by transthoracic echocardiography was difficult. In addition, there were no obvious findings of coronary artery malperfusion on CT because a detailed imaging evaluation in the aortic root was difficult.
Furthermore, the patient was experiencing severe hemodynamic instability, which precluded lengthy preliminary investigations. Immediate surgical intervention was required, and further intraoperative assessment conclusively excluded the diagnosis of AAD. Rather, a small perforation was located on the free-wall of the left ventricle having occurred as a complication of posterior wall myocardial infarction with presentations mimicking aortic dissection. The rupture was successfully repaired using a tissue-sealing sheet, and the patient was discharged several days later.
This case demonstrates that other cardiac events should not be automatically excluded in instances of suspected aortic dissection based solely on imaging findings. Furthermore, in cases of critical hemodynamic instability caused by pericardial hematoma, immediate surgical intervention supports further diagnostic investigation and provides the opportunity for effective treatment.
2 CASE REPORT
A 72-year-old male patient with a medical history of untreated hypertension presented to the emergency department (ED) complaining of sudden onset chest pain and dyspnea persisting for 30 min. The pain was described as “tearing” and substernal. Immediately after arrival, the patient temporarily lost consciousness. His condition worsened in the ED, and his BP and oxygen saturation were decreased to 60/35 mmHg and 88%, respectively. Jugular vein distension was found on physical examination, but cardiac examination revealed normal heart sounds. Bedside transthoracic echocardiography demonstrated cardiac tamponade with massive hematoma, hypokinesis in the inferior to lateral wall of the left ventricle and mild aortic insufficiency; however, a detailed examination was not possible because of the pericardial hematoma. At the same time, as far as the observation made, no dilatation of the ascending aorta was detected and there was no flap in the ascending aorta. There was no time to take a chest X-ray.
Emergency CT revealed cardiac tamponade (Figure 1A), a crescent-shaped filling defect around the ascending aorta (Figure 1B), and hypoperfusion in the posterior wall of the LV (Figure 1C). On the contrary, contrast of the infarcted ventricular wall and pericardial cavity, which are the characteristics of myocardial infarction, could not be detected. A 12-lead ECG showed ST segment elevation in the inferior leads (Figure 1D). Therefore, AAD (type A) complicated with coronary malperfusion was suspected. Initial laboratory data indicated mild inflammatory changes, mild coagulation abnormalities, and myocardial ischemia according to the following blood test results: white blood cell count, 28 × 106/L; fibrinogen, 666 mg/dL; D-dimer, 2.5 µg/ml; N-terminal pro b-type natriuretic peptide (NT-proBNP), 357 pg/ml; creatine phosphokinase, 174 U/L; creatine phosphokinase-MB, 25 U/L; and troponin I, 10806 ng/ml; however, these results had not yet been obtained at the ED. The patient had severe hemodynamic instability and was transferred to an operating room for emergency surgical intervention.
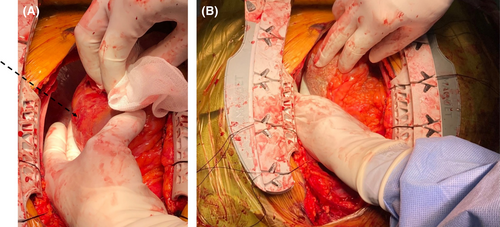
The preliminary diagnosis of AAD was not supported by surgical findings. Intraoperative transesophageal echocardiography did not detect a localized tear in the visible aorta. After median sternotomy, a massive hematoma was visible around the heart and the ascending aorta. The removal of the hematoma uncovered a normal aortic wall with no evidence of dissection or hematoma. After the establishment of cardiopulmonary bypass with the femoral artery cannulation and the superior vena caval drainage, direct echocardiography confirmed no AAD-related findings. Further inspection of the pericardium exposed a small perforation in the posterior free-wall of the LV (Figure 2A), which was repaired with TachoSil® tissue-sealing sheet (Figure 2B).
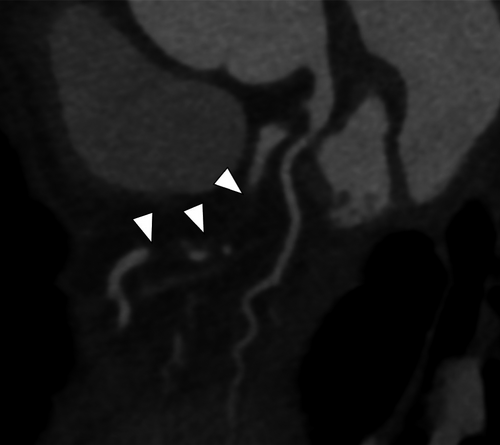
The patient experienced no postoperative complications and recovered with the administration of optimal medication for myocardial infarction, and sufficient rehabilitation. Postoperative coronary CT revealed the occlusion of the obtuse marginal branch (Figure 3A), considered to be the cause of the infarction leading to free-wall rupture. On the 9th postoperative day, he was discharged with the condition of NYHA class II. He came to our hospital for the checkup in a week, which showed no significant concerns. He has been checked as an outpatient for a few months since then. The patient has remained free from any cardiovascular events for 6 months after surgery.
3 DISCUSSION
Aortic dissection is frequently confused with myocardial ischemia resulting in delayed or incorrect diagnosis as well as inappropriate treatment with antithrombotic agents.1, 2 Symptoms of AAD vary greatly and have an array of presentations mimicking other disorders.1 A serious complication of AAD is coronary malperfusion, often misdiagnosed as myocardial infarction.3 Further, approximately 20% of patients with type A dissection present ECG evidence of acute ischemia or AMI.4 Motion abnormalities of the LV regional wall caused primarily by low coronary perfusion are seen in 10%−15% of patients with AAD.2 Therefore, the clinical distinction of AAD from ACS may be difficult.3 In the current case, cardiac tamponade and the formation of a crescentic rim around the ascending aorta further hindered preliminary differentiation between AAD and LV free-wall rupture (Figure 1e). Our case may be very rare because there has been no case of LV rupture mimicking aortic dissection reported in the literature.
The contrast CT imaging modality is commonly used for AAD detection and is under constant development for artifact elimination.4 However, misdiagnosis of AAD by CT is still possible. Other examples of diagnostic confusion arising from CT include the superior pericardial recess6 and the curvilinear configuration of thickened pericardium, which may mimic the intimal flap of an aortic dissection on transverse sections.7 In this case, CT images revealed a clearly delineated contrast-blood layering spreading from the aortic valve through the ascending aorta and bearing close resemblance to the crescent-shaped filling defect characteristic of AAD. In addition, neither contrast of the infarcted ventricular wall nor contrast of the pericardial cavity, which are characteristics of myocardial infarction, could be detected. However, in the present case, there was no obvious relationship between the ECG and the coronary artery malperfusion on CT. Further, even though the image quality was poor, echocardiography showed neither dilatation of the ascending aorta nor a flap within the ascending aorta. If we had had time to evaluate the patient calmly, we would have noticed this discrepancy.
Often, symptom overlap with AAD is evident in CT, and rescanning suspicious areas using narrower collimation or transesophageal echocardiography is recommended to confirm the diagnosis.7 Raymond et al. demonstrated that motion artifacts arising from non-electrocardiogram synchronized CT imaging mimic AAD such that an AAD diagnosis could not be confirmed or excluded by this method.8 Multidetector CT may be effective for a structural evaluation of the ascending aorta and helps create a more detailed image by synchronizing the imaging acquisition with cardiac pulsation. However, this modality requires an amount of time for completion that may not be available in critical cases like the present case. Accordingly, the effectiveness and accuracy of these imaging methods is largely situational and may need to be adapted on a case-by-case basis. Regardless, the importance of careful analysis based on closely spaced consecutive sections for the confirmation or elimination of AAD diagnosis cannot be overemphasized.
In cases of cardiac tamponade complications, accurate diagnosis via transthoracic echocardiography may prove to be especially challenging. Moreover, there is no opportunity for lengthy diagnostic procedures when a patient is in a critical state of hemodynamic instability. In our case, treatment was prioritized over searching for the tamponade origin. Open thoracotomy drainage rather than drainage via needle puncture is recommended because the former is more effective9; in our case, the open approach helped successful investigation and treatment of the cardiac tamponade.
Understanding the frequencies and causes of false-positive activation in patients with suspected acute aortic syndrome provides an opportunity for improved clinical care.7, 8 The advancement of examination protocols for a better distinction between AAD and other potentially life-threatening cardiac disorders requires the development of novel methodologies and the refinement of existing approaches.
4 CONCLUSION
The role of CT in AAD diagnosis is established and effective; however, this case illustrates the importance of artifacts and false positives during patient assessment. Transesophageal echocardiography or electrocardiogram synchronized multidetector CT imaging may provide a more definitive diagnosis of aortic pathology; however, such approaches are not feasible when time is limited. In such cases, a multifaceted approach is desirable, assuming as many exclusionary diagnoses as possible for an accurate diagnosis. In cases of critical hemodynamic instability related to pericardial hematoma, open thoracotomy drainage is recommended for treatment and subsequent conclusive diagnosis.
AUTHOR CONTRIBUTIONS
KS: analyzed and interpreted the data, and drafted and revised the manuscript. KM: analyzed, drafted, and revised the manuscript. All authors: provided final approval of the paper and agreed to be accountable for the integrity of the case reports.
ACKNOWLEDGMENTS
We thank the Japan Medical Communication staff for reviewing and editing this manuscript. We also thank our colleagues for their guidance and constructive feedback.
CONFLICT OF INTEREST
None to disclose.
ETHICAL APPROVAL
All procedures were performed according to the tenets of the Helsinki Declaration. The patient provided written consent for their clinical data to be used for scientific presentations or publications. This case report was approved by the Institutional Review Board of Aichi Medical University Hospital.
CONSENT
Written consent was obtained from the patient for publication of this case and use of the images.
PRESENTATION AT A MEETING OR SCIENTIFIC SYMPOSIUM
None.