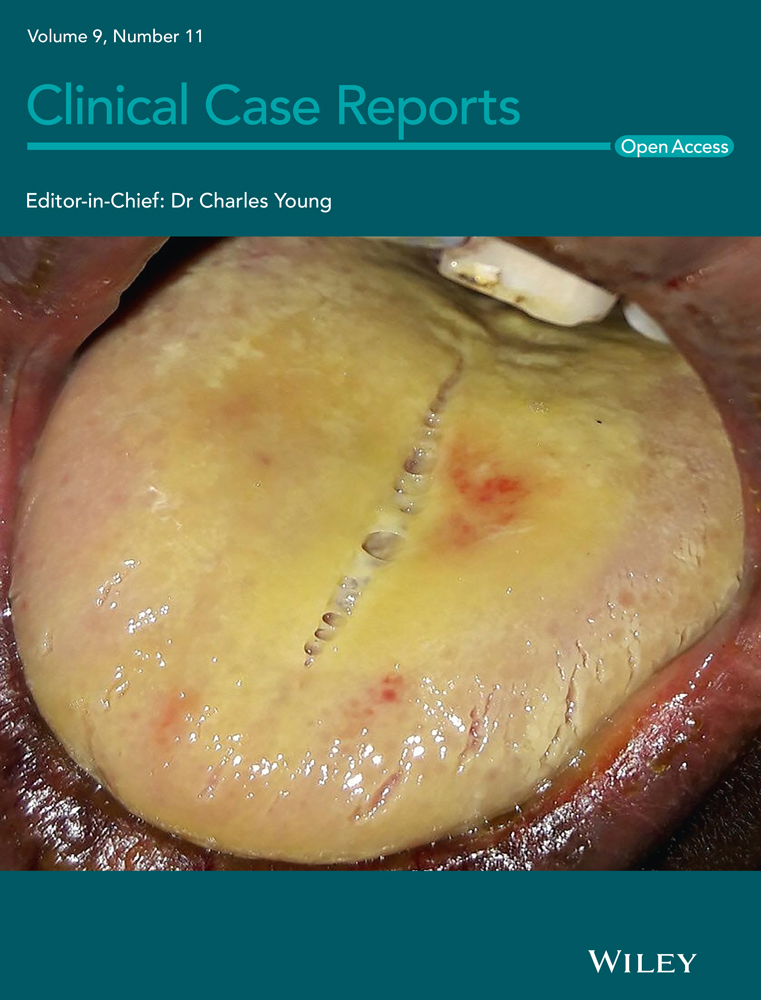
Cribriform adenocarcinoma of the tongue with cervical lymph nodes involvement: Report of an extremely rare tumor and focus about differential diagnosis with polymorphous adenocarcinoma
Funding information
No funding to declare
Abstract
Cribriform adenocarcinoma is a rare tumor that arise in tongue and minor salivary glands. Previously, only 56 case have been described. In this paper, we report a case of a 83-year-old man and we focus about the differential diagnosis with polymorphous adenocarcinoma.
1 INTRODUCTION
Cribriform adenocarcinoma of the tongue and minor salivary glands (CATMSG) is a relatively new salivary gland tumor that was originally described in 1999 by Michal et al.1 as a distinctive type of tumor occurring typically in the posterior tongue and retromolar region with concurrent metastases in lateral neck lymph nodes, under the name of “cribriform adenocarcinoma of the tongue”; then, in 2005, it was classified by the World Health Organization of Tumors as a variant of polymorphous low-grade adenocarcinoma (PLGA).2 It is currently accepted as a provisional entity in the WHO classification since Skalova et al.3 renamed it “cribriform adenocarcinoma of the tongue and minor salivary glands” because they found that it was different in term of histological features and biological behavior from PLGA. CATMSG is a rare tumor that affects equally men and women, more often Africans and African Americans, and occurs primarily in the posterior tongue, but there are cases of CAMSG described from the soft palate, retromolar buccal mucosa, lingual tonsils, upper lip, epiglottic.4 In this paper, we report the case of a 83-year-old man with CATMSG located in the posterior area of the tongue with cervical lymph node metastases at presentation, and we focused about the discussed differential diagnosis between CATMSG and PLGA.
2 CASE PRESENTATION
An 83-year-old diabetic man was referred to our Otolaryngology Department relating three months of pain and discomfort when eating. He denied a history of alcohol or tobacco use. Family history of prior head and neck cancer was negative. A flexible fiberoptic laryngoscopy showed irregular profile of the mucosa at the base of the tongue. On physical examination, firm left neck masses at level II were palpable. He underwent an enhanced magnetic resonance imaging (MRI) which revealed an expansive mass (37 × 33 mm) of T2 hyperintensity and heterogeneous enhancement at the base of the left tongue, involving the body of the tongue, the anterior belly of the digastric muscle, the palatoglossal muscle, and imprinting the homolateral parapharyngeal space; it also medially displayed the ipsilateral palatine tonsil. The mass extended along the lingual tonsil up to the level of the left vallecula. The MRI also showed a mass (10 × 17 mm) of the emitongue on the left side, left level II lymphadenopathies, and a nodular mass (60 × 42 mm) of the right hemithyroid displacing the trachea to the contralateral side (Figure 1). A biopsy of the base of the tongue was performed. Architecturally, the tumor showed cribriform and solid structures, which contained solid nests appearing in glomeruloid areas and collagen-rich stroma. Cytologically, the tumor was composed of one cell type; the chromatin was dispersed, mitotic figures were rare, and necrotic foci were absent (Figure 2). The overlying squamous epithelium was intact. The neoplasia was present on surgical margins of resection. Immunohistochemical studies showed the tumor cells to be positive for CK AE1/AE3, CK 7, S100 protein, and CD117. They were negative for CK20, TTF1, DOG1, and thyroglobulin. A diagnosis of CATMSG was established.
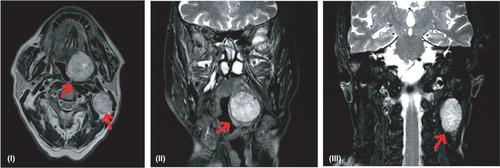
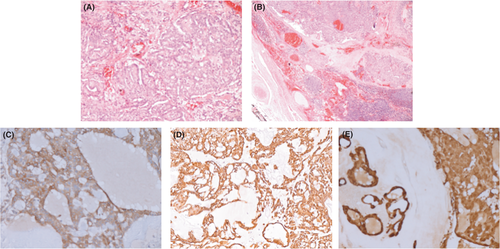
Five days after the biopsy, the patient underwent a base tongue mucosectomy with bilateral selective neck dissection. The final pathology confirmed the diagnosis of CATMSG; it also showed extracapsular tumor extension within level II lymph node metastases without extranodal spread, and the presence of a parathyroid adenoma on the right hemithyroid. According to the 8th edition of UICC/AICC, the staging of the disease was pT3, N2b, and R1. The patient subsequently received adjuvant radiation therapy of the tumor bed and cervical lymph node basin. The patient is alive and without signs of disease after 3 years.
3 DISCUSSION
To our knowledge, only 56 five cases of CATMSG have been previously reported.1, 3-16 Since it was firstly described in 1999 by Michael et al, the perception of this rare tumor has been changed over the years and is currently accepted as a distinct entity. The main differential diagnosis is with PLA, of which CATMSG has been considered a variant for years. PLA is an indolent salivary gland carcinoma characterized by architectural diversity; it occurs in minor salivary glands, in particular in the palate (60% of cases), and it shows an excellent prognosis with a 10 year disease specific survival of 95%. Architecturally, it presents a cytologic uniformity (the tumor is composed of one single type of tumor cells characterized by monotonous pale nuclei with marked chromatin clearing resembling that of papillary thyroid carcinoma, and an highly variable architectural patterns of different proportions, in contrast with CATMSG, which is cytologically monomorphous tumor). Another interesting feature of CAMSG is the nuclear similarity to papillary carcinoma of the thyroid. This feature is not observed to a greater extent in PLGA. S100, CK7, and SOX10 are diffusely sand strongly positive, while p63 is variably positive; p40 is always negative, while EMA, SMA, GFAP, MSA, and GATA3 are positive in almost 15% of cases.
4 CONCLUSION
Cribriform adenocarcinoma of the tongue and minor salivary glands is a relatively new entity in salivary glands tumors that, due to its biological, histological, and himmunoistochemical features, should be considered as a distinct tumor entity from PLGA. To our knowledge, only 56 five cases of CATMSG have been previously described. Also the description of individual cases should be encouraged, helping scientific community to better understand the behavior and the therapeutic options in the management of this rare tumor.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.
AUTHOR CONTRIBUTIONS
Pietro De Luca: Original idea, manuscript writing, literature review. Domenico Tassone and Luca de Campora: literature review and English editing. Matteo Simone: English Editing and table editing. Pasquale Marra and Vito Colacurcio: manuscript writing and table editing. Leopoldo Costarelli: manuscript revision, table revision, and final approval. Alfonso Scarpa: manuscript writing and editing. Claudio Viti: original idea and critical revision. Angelo Camaioni: original idea, critical revision, and final approval.
CONSENT
The patient signed an informed consent to publication.
Open Research
DATA AVAILABILITY STATEMENT
Availability of data and material. No data available.