Eslicarbazepine-induced severe hyponatremia resulted in generalized tonic-clonic seizure
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abstract
Although eslicarbazepine is an anti-seizure medication, it may result in seizure worsening if its use is complicated by severe hyponatremia, even long time after commencing this medication. The treatment is a replacement for another ASM.
Abbreviations
-
- ASM
-
- Anti-seizure medication
-
- CT
-
- Computed tomography
-
- ESL
-
- Eslicarbazepine
-
- GTC
-
- Generalized tonic-clonic
-
- SIADH
-
- Syndrome of inappropriate antidiuretic hormone secretion
1 INTRODUCTION
Eslicarbazepine (ESL) is an anti-seizure medication used to treat focal seizures. Although mild hyponatremia is a well-known side effect of ESL, late and severe hyponatremia causing generalized tonic-clonic (GTC) seizure as an adverse effect is quite rare. We present a case of GTC seizure provoked by ESL-induced severe hyponatremia.
Eslicarbazepine acetate is an oral anti-seizure medication, a member of the dibenzazepine family along with carbamazepine and oxcarbazepine, which blocks the voltage-gated sodium channels in central nervous system and is currently used to treat focal-onset seizures in adults.1 The safety and efficacy of ESL were evaluated in multiple studies.1 The commonly reported adverse effects were nausea, vomiting, dizziness, drowsiness, headache, and vertigo.1 Hyponatremia is generally defined by serum sodium level below 135 mEq/L. It can manifest in a broad range of symptoms such as nausea, vomiting, and headache. Seizures may happen in severe cases.2
Oxcarbazepine and carbamazepine are the most known anti-seizure medications (ASMs) inducing hyponatremia. The mechanism behind hyponatremia induced by ASMs is still not fully clear. However, there is evidence that ASMs can partially stimulate vasopressin 2 receptor and aquaporin 2 pathway causing antidiuretic effect, which may lead to hyponatremia.3 The syndrome of inappropriate antidiuretic hormone secretion (SIADH) has been also proposed as a possible mechanism, especially in relation to dibenzazepine family.4 Hyponatremia (<135 mEq/L) was reported in 6.1%, 4.8%, and 6.6% of patients treated with ESL 400, 800, and 1200 mg, respectively. However, severe hyponatremia (<120 mEq/L) was uncommon.5 Hyponatremia led to treatment discontinuation in less than 1% of the patients taking eslicarbazepine acetate.5 GTC seizure as an adverse effect of ESL-induced hyponatremia was rarely reported.
2 CASE REPORT
A 53-year-old gentleman, known to have diabetes, hypertension, stroke, and focal epilepsy predating his stroke, presented to our hospital after an episode of witnessed, generalized tonic-clonic seizure at home. The patient has a long-standing history of focal epilepsy since childhood for which he was prescribed carbamazepine (complicated by asymptomatic moderate hyponatremia) then changed to eslicarbazepine 400 mg AM and 800 mg PM for the past 2 years. Other home medications for the last 2 years included metformin, insulin glargine, aspirin, rosuvastatin, and valsartan with no recent change in his medications. According to the family, he had a sudden onset of generalized tonic-clonic seizure for around three minutes with fecal and urinary incontinence and postictal confusion. The patient did not experience any prodromal symptoms or abnormal movements before the event.
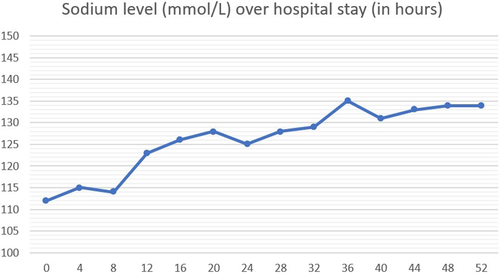
On admission, the patient was in postictal state with confusion and agitation. Soon, he developed another episode of GTC seizure, which was aborted by IV lorazepam. Physical examination showed blood pressure of 150/86 mmHg and normal heart rate, respiratory rate, and oxygen saturation. The patient was confused, but otherwise general examination was unremarkable. Neurologic examination revealed only mild left upper limb weakness (which was described in previous notes as a sequela of his past stroke). Laboratory workup showed severe hyponatremia (sodium 112 mmol/L), with low serum osmolality (Table 1). Computed tomography (CT) of head showed no acute insults when compared with a previous CT. GTC seizure was attributed to severe hyponatremia in setting of probable SIADH (urine sodium was not sent), which is most likely induced by ESL. ESL was discontinued, and the patient was started on hypertonic saline infusion for the next few hours. Sodium level was stabilized (Figure 1) and no more seizures were detected. Lacosamide was then introduced to substitute ESL for the chronic focal epilepsy, then the patient was discharged home after 3 days. Follow-up in the clinic 3 weeks later demonstrated serum sodium 137 meq/L with no more episodes of seizure.
| Detail | Value w/Units | Normal Range |
|---|---|---|
| Urine Osmolality | 224 mmol/kg | 150–1150 |
| Serum Osmolality | 246 mmol/kg | 275–295 |
| Calcium | 2.12 mmol/L | 2.15–2.50 |
| Phosphorus | 1.18 mmol/L | 0.80–1.50 |
| Magnesium | 0.69 mmol/L | 0.70–1.00 |
| White blood cell count | 13.3 × 103/μl | 4.0–10.0 |
| Hemoglobin | 13.5 gm/dl | 13.0–17.0 |
| Urea | 3.2 mmol/L | 2.5–7.8 |
| Creatinine | 69 μmol/L | 62–106 |
| Sodium | 112 mmol/L | 133–146 |
| Potassium | 5.3 mmol/L | 3.5–5.3 |
| Bicarbonate | 19 mmol/L | 22–29 |
| Glu Fasting | 6.5 mmol/L | 3.3–5.5 |
| Thyroid-stimulating hormone | 1.28 mIU/L | 0.30–4.20 |
| Free T4 | 19.4 pmol/L | 11.0–23.3 |
Note
- Urine sodium was not sent!
3 DISCUSSION
Many underlying causes have been described behind GTC seizures such as traumatic brain injury, ischemic or hemorrhagic strokes, electrolyte disturbances including hyponatremia, and medication's side effects. Hyponatremia has been reported in patients treated with ESL (0.6%–1.3%), but remarkably less compared with oxcarbazepine and carbamazepine.6, 7 Older age, high serum level of anti-seizure medications, anti-seizure polypharmacy, and accompanying sodium losing medications might increase the risk.8 ASMs-induced hyponatremia evolves slowly and gradually over few months and usually remains asymptomatic until serum Na level declines to less than 120 meq/L.9 Nausea, vomiting, headache, confusion, restlessness, and seizures are common symptoms of hyponatremia. Several studies have described ESL-associated hyponatremia.10-16 A post hoc exploratory analysis examined the frequency of hyponatremia and related symptoms in clinical trials of ESL in adults with focal seizures and showed that <3.3% of patients taking ESL had a minimum post dose sodium level ≤125 mEq/L and less than 6% had a hyponatremia-related symptom. Convulsions and focal seizures with secondary generalization were described more frequently in patients with serum sodium level below 125 mEq/L.17 Furthermore, the latter study reported increased seizure frequency and severity with ESL-associated hyponatremia, and secondary generalization (3.3% of patients) was only observed in mild to moderate hyponatremia subgroups.17
In our case, the patient has had a history of long-standing focal epilepsy which was controlled with ESL, but he came with GTC seizure for the first time which could be primarily generalized or secondary to a focal seizure. History was unremarkable for any trauma or recent change in his medications. Physical examination did not suggest any new neurologic findings as well. Laboratory workup revealed severe hyponatremia (sodium = 112 mmol/L), which was considered the provoking cause of the GTC seizure, while other blood tests were unremarkable. The sudden worsening in seizures was attributed to the hyponatremia. The suggested mechanism of hyponatremia related to dibenzazepine is SIADH, which was consistent with our patient's labs (low serum sodium, low serum osmolality compared to relatively high urine osmolality in combination with normal renal function, glucose, and TSH). The improvement and stabilization of the clinical condition after treatment with hypertonic saline and holding the offending medication also support the diagnosis.
4 CONCLUSION
ESL-induced hyponatremia should be kept in mind as a potential cause of seizure worsening in patients with otherwise well-controlled chronic focal epilepsy. Physicians should recognize symptoms of hyponatremia in patients receiving ESL such as nausea, tiredness, irritability, confusion, muscle weakness/spasms, and more frequent or more severe seizures even after a long-time use of ESL. Moreover, regular monitoring of serum sodium level might be reasonable in patients receiving ESL.
ACKNOWLEDGMENT
The authors would like to thank the patient for allowing us to share his details with the medical community. Additionally, we acknowledge the Qatar National Library for funding the open access fees of this publication.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHORS CONTRIBUTIONS
MBH conceptualized and wrote the original draft; ME, EA, KA, and SS participated in literature review and edited the manuscript; KA prepared the table and the graph; NH reviewed the final manuscript; MA revised and edited the manuscript.
CONSENT
Published with written consent of the patient.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.