Minimally invasive mitral valve repair and ablation of concomitant atrial fibrillation in a patient with severe hemophilia A
Abstract
Minimally invasive mitral valve repair and ablation of atrial fibrillation, combined with FVIII level-controlled replacement therapy, can be safely performed in patients with severe hemophilia.
1 INTRODUCTION
Cardiac surgery in patients with hemophilia remains a serious problem due to the risk of bleeding complications. Here, we report successful treatment of mitral valve insufficiency and concomitant atrial fibrillation in a patient with severe hemophilia A.
Hemophilia A is a rare X-linked bleeding disorder caused by mutations in the gene that encodes factor VIII (FVIII) with a prevalence of 1/5000 live male births.1 Disease severity is based on FVIII levels in the blood: the severe form is defined as a factor level <1% of normal, the moderate form as a factor level of 1%-5%, and the mild form as a factor level between 5% and 40%.2 Cardiac surgery in patients with severe hemophilia remains a serious problem due to the risk of large intra- and postoperative blood loss, absence of evidence-based guidelines regarding factor replacement therapy, and anticoagulation treatment after valve intervention using cardiopulmonary bypass. Here, we report the successful, minimally invasive treatment of mitral valve insufficiency and concomitant atrial fibrillation (AF) in a patient with severe hemophilia A.
2 CLINICAL CASE
A 50-year-old man (62 kg body weight) with a history of hemophilia A entered our clinic with complaints of dyspnea during exercise, palpitations, and weakness. The FVIII level was 0.7% when hemophilia was first diagnosed. The patient had mitral valve prolapse with moderate regurgitation for over 12 years. A persistent form of AF was diagnosed a year prior to presentation at our clinic; the patient underwent an unsuccessful attempt of electrical cardioversion.
Upon admission, the patient had deformation and limited mobility in the elbow and knee joints (due to recurrent hemarthrosis), apex systolic murmur, and an abnormal heart rhythm with a heart rate ranging from 55 to 160 beats per minute (bpm). An ECG revealed AF with a ventricular rate of 110 bpm. Transthoracic echocardiography (TTE) showed both mitral valve leaflets prolapse with severe regurgitation (vena contracta of 0.8 cm, effective regurgitant orifice area of 45 mm2), significant enlargement of the left atrium (volume of 270 mL), left ventricular diastolic volume of 170 mL, and ejection fraction of 50%. The FVIII level was 8.7% after replacement therapy with 1000 IU FVIII (16 IU/kg) in 1 day. Inhibitory antibodies toward FVIII were not detected. CHA₂DS₂-VASc Score was 1.
A perioperative coagulation management strategy for this patient was developed. Thirty minutes preoperatively, he was administered 4000 IU (65 IU/kg) of FVIII concentrate and reached a 109% level of FVIII activity. Surgery was performed using a right anterolateral minithoracotomy (approximately 5 cm long) in the fifth intercostal space with femoral artery and vein cannulation. Tranexamic acid (1 g/h) was infused, beginning with the skin incision to the completion of the procedure. Prior to cannulation, a heparin bolus of 300 IU/kg was administered and resulted in an activated clotting time ≥400 s while on cardiopulmonary bypass, during which FVIII replacement therapy was not performed. Myocardial protection was performed using antegrade cardioplegia (Custodiol, Dr F Kӧhler Chemie). The mitral valve was repaired by quadrangular resection of the posterior leaflet with sliding plasty and decalcification of the posterior annulus, and implantation of a flexible band (40 mm, CardioMed; Figure 1).
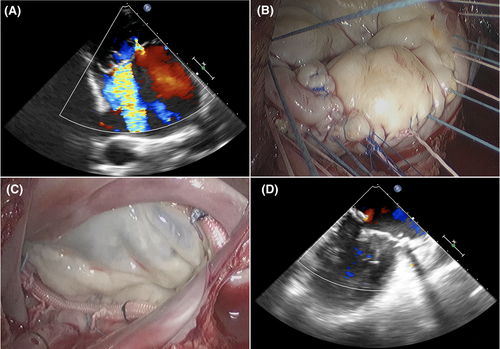
The left and right atrial ablation was performed according to the Cox maze IV pattern3 using the nitrous oxide-based CryoICE Probe (AtriCure Inc). The left atrial appendage was excluded using a double-layer 3/0 polypropylene suture. After weaning the patient from bypass, protamine was administered to inactivate heparin. The cardiopulmonary bypass and cross-clamping times were 110 and 90 minutes, respectively. After inactivating heparin, 2000 IU (32 IU/kg) of FVIII was injected (Figure 2).
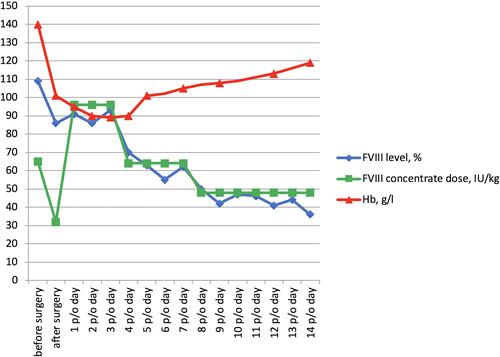
The patient was extubated 10 hours after being transferred to the intensive care unit (ICU). The early postoperative period was complicated by moderate heart failure; therefore, the patient received inotropic therapy during the first day. An episode of supraventricular tachycardia was noted in the ICU and cured by amiodarone infusion. The total drainage losses were 700 mL (first day) and 400 mL (second day). The patient received 500 mL of fresh frozen plasma on postoperative day 1. The minimum hemoglobin level was 89 g/L, and blood transfusions were not required. The patient was transferred from the ICU to the general ward on postoperative day 3. Postoperatively, no anticoagulant or antiplatelet therapy was prescribed.
Postoperative replacement therapy was maintained as follows. A bolus of 3000 IU (48 IU/kg) FVIII was administered 12 hours after the first injection, then 3000 IU of FVIII was continued every 12 hours on days 1-3, 2000 IU (32 IU/kg) every 12 hours on days 4-7, and 3000 IU (48 IU/kg) once per day on days 8-14. Beginning on postoperative day 15, 1000 IU FVIII (16 IU/kg) was administered every other day. The FVIII levels were measured twice a day during the first 3 days postsurgery and once every day until discharge.
Transthoracic echocardiography at the time of discharge revealed trivial mitral regurgitation. A 24 hours Holter monitoring device confirmed a sinus rhythm with an average daily heart rate of 72 bpm and absence of AF. The patient was discharged 15 days after surgery. The hemoglobin level at discharge was 119 g/L. Bisoprolol and amiodarone (2.5 and 200 mg/d, respectively) were prescribed, and amiodarone was discontinued 3 months later due to no AF paroxysms and sinus rhythm on 24-hours Holter monitoring.
Two years postsurgery, the patient was classified with New York Heart Association class I heart failure, had sinus rhythm without AF paroxysms, and had no bleeding or thrombotic complications. Transthoracic echocardiography revealed trivial mitral insufficiency and a left ventricle ejection fraction of 60%.
3 DISCUSSION
Heart valve surgery in patients with severe hemophilia remains a significant challenge due to severe changes in the hemostatic system and increased risk of bleeding complications. There are no guidelines for the perioperative management of hemophiliacs undergoing cardiac surgery. Current practice is based on expert opinions, case reports, or small case series. In most cases, biological prostheses are used for valve replacement in hemophiliacs. Since life expectancy of patients with hemophilia has increased, the possibility of degeneration of biological prostheses and need for further surgery may carry an even greater risk to the patient. In the case of mitral insufficiency in young patients, valve repair is preferable to valve replacement as it potentially avoids the need for short-term anticoagulation treatment. The literature presents a few cases of mitral valve reconstruction in hemophilia A patients.4-7 Only one describes a minimally invasive mitral valve repair with concomitant atrium ablation.8 Minimally invasive approach for mitral valve surgery has advantages over standard sternotomy, which may be especially important in patients with bleeding disorders. It reduces intra- and postoperative bleeding resulting in a decreased need for transfusion. Furthermore, ventilation time and ICU or hospital stays are shortened.9, 10
Factor replacement therapy is crucial for successful surgical treatment of hemophiliac patients. Patients with a preplanned coagulation management strategy can successfully undergo complex cardiac surgery as patients without hemostatic disturbances. According to the recommendations of the World Hemophilia Federation,11 the following FVIII activity levels should be achieved in patients undergoing major surgical procedures: before surgery, 80%-100%; postoperative days 1-3, 60%-80%; postoperative days 4-6, 40%-60%; and postoperative days 7-14, 30%-50%. Other recommendations for patients undergoing coronary artery bypass grafting suggest that FVIII levels should be 80%-100% until postoperative day 5 and above 50% after that.12 In our case, we maintained FVIII levels above 80%-100% during the first 3 days postsurgery and 50%-100% until postoperative day 7. This strategy allowed us to prevent hemorrhagic complications in the postoperative period.
Although patients with hemophilia are relatively protected from thrombosis, there is still a risk of thromboembolism, especially in the presence of concomitant AF. According to frequently cited antithrombotic treatment algorithms for hemophiliacs with nonvalvular AF, antithrombotic regimens are based on baseline FVIII levels and risk of thromboembolic complications.13 Low-dose aspirin therapy is indicated in patients with high stroke risk (CHA₂DS₂-VASc Score ≥2) with baseline FVIII levels of 1%-10% and patients with baseline FVII of 10%-30%. Long-term anticoagulation treatment with vitamin K antagonists is indicated when factor levels are above 30%. In the presence of AF in hemophiliacs undergoing cardiac surgery, antithrombotic treatment remains unclear. As anticoagulant therapy in patients with severe hemostasis disorders is extremely undesirable, we believe that concomitant ablation and left atrial appendage closure should be considered to restore sinus rhythm and reduce thromboembolic risk.
In our case, although the left atrium was significantly dilated, the patient still had a chance of sinus rhythm restoration due to his young age, nonrheumatic etiology of mitral valve disease, and relatively short history of arrhythmia. We abandoned the rate-control approach and performed ablation, which was successful and allow us not to prescribe anticoagulants and antiplatelets at all.
4 CONCLUSION
This case demonstrates that minimally invasive mitral valve repair can be safely performed in patients with hemophilia. Using a comprehensive approach, including a FVIII level-controlled replacement therapy, a minimally invasive valve repair, and sinus rhythm restoration, may minimize the risk of complications in the perioperative and postoperative periods in patients with severe hemophilia A.
ACKNOWLEDGMENTS
None
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
ABP: approved the final manuscript; RS: wrote the manuscript; AK: wrote the manuscript; NL: wrote the manuscript; VS: reviewed the manuscript; IK: reviewed the manuscript; AM: reviewed the manuscript; AA: wrote the manuscript; AP: wrote the manuscript and prepared figures.
ETHICAL APPROVAL
Approved by the Institutional ethics committee. Published with written consent of the patient.