Identification of an Alepterolic Acid Derivative as a Potent Anti-Breast-Cancer Agent via Inhibition of the Akt/p70S6K Signaling Pathway
Nina Wang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorLei Zhang
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorJunjie Yu
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorKaili Chang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorMinghui Fan
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zi Liu
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorProf. Dr. Liang Ma
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianguo Cao
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guozheng Huang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorNina Wang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorLei Zhang
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorJunjie Yu
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorKaili Chang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorMinghui Fan
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zi Liu
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorProf. Dr. Liang Ma
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianguo Cao
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guozheng Huang
Department of Chemical Biology and Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma'anshan, Anhui, 243002 China
College of Life Sciences, Shanghai Normal University, Shanghai, 201418 P.R. China
Search for more papers by this authorAbstract
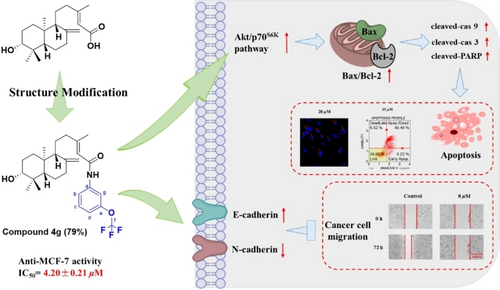
Alepterolic acid is a diterpene occurring in the fern Aleuritopteris argentea with potential biological activity that warrants further structural modification. In the present work, sixteen alepterolic acid derivatives were synthesized and evaluated for their anticancer activities. Among them, N-[m-(trifluoromethoxy)phenyl] alepterolamide displayed comparable activity (IC50=4.20±0.21 μM) in MCF-7 cells. Moreover, mechanistic investigations indicated this compound was significantly capable of diminishing cell proliferation and viability of MCF-7 cells. After treatment with N-[m-(trifluoromethoxy)phenyl] alepterolamide, a significant increase in cleaved caspase-9, cleaved caspase-3, cleaved poly (ADP-ribose) polymerase (PARP) and Bax/Bcl2 ratio were observed in MCF-7 cells, leading to caspase-dependent apoptotic pathways. Further studies showed this compound promoted cellular apoptosis and inhibited migration in MCF-7 cells via modulation of the Akt/p70S6K signaling pathway. All these results revealed the potential of N-[m-(trifluoromethoxy)phenyl] alepterolamide as an appealing therapeutic drug candidate for breast cancer.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202301248-sup-0001-misc_information.pdf3.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. D. Kochanek, B. L. Smith, Natl. Vital Stat. Rep. 2004, 52, 1–47.
- 2R. L. Siegel, K. D. Miller, H. E. Fuchs, A. Jemal, Ca-Cancer J. Clin. 2022, 72, 7–33.
- 3A. G. Waks, E. P. Winer, JAMA 2019, 321, 288–300.
- 4A. L. Harvey, R. Edrada-Ebel, R. J. Quinn, Nat. Rev. Drug Discovery 2015, 14, 111–129.
- 5W. Ge, X. Hao, F. Han, Z. Liu, T. Wang, M. Wang, N. Chen, Y. Ding, Y. Chen, Q. Zhang, Eur. J. Med. Chem. 2019, 166, 445–469.
- 6X. Feng, D. Liao, D. Liu, A. Ping, Z. Li, J. Bian, J. Med. Chem. 2020, 63, 15115–15139.
- 7S. Xu, H. Yao, Y. Qiu, M. Zhou, D. Li, L. Wu, D. H. Yang, Z. S. Chen, J. Xu, J. Med. Chem. 2021, 64, 17346–17365.
- 8C. Wei, H. Zou, T. Xiao, X. Liu, Q. Wang, J. Cheng, S. Fu, J. Peng, X. Xie, J. Fu, J. Cell. Mol. Med. 2021, 25, 10101–10110.
- 9Y. Gautam, S. Das, H. Khan, N. Pathak, H. Iqbal, P. Yadav, V. K. Sirohi, S. Khan, D. S. Raghuvanshi, A. Dwivedi, D. Chanda, K. Shanker, F. Khan, R. Konwar, A. S. Negi, Bioorg. Med. Chem. 2021, 42, 116252.
- 10X. X. Jin, Y. N. Mei, Z. Shen, J. F. Zhu, S. H. Xing, H. M. Yang, G. Liang, X. H. Zheng, Phytomedicine 2022, 101, 154087.
- 11H. Liang, W. Wang, F. Zhu, S. Chen, D. Liu, C. Sheng, Bioorg. Med. Chem. 2022, 65, 116793.
- 12J. Qin, X. Sun, Y. Ma, Y. Cheng, Q. Ma, W. Jing, S. Qu, L. Liu, Bioorg. Med. Chem. 2022, 55, 116594.
- 13F. Yang, K. Xu, S. Zhang, J. Zhang, Y. Qiu, J. Luo, G. Tan, Z. Zou, W. Wang, F. Kang, Bioorg. Med. Chem. 2022, 66, 116809.
- 14L. Zhang, X. Qin, C. Lian, J. Liu, Bioorg. Med. Chem. 2023, 81, 117188.
- 15G.-M. Zhang, S.-Y. Dong, X.-C. Zhang, S.-G. Lu, Pteridology in the New Millennium, NBRI Golden Jubilee Vol, Kluwer Academics Publishers, 2003, pp. 143–151.
10.1007/978-94-017-2811-9_11 Google Scholar
- 16Z. Cheng, S. Wu, Acta Bot. Yunnan. 1997, 19, 75–78.
- 17H. Ageta, K. Awata, Y. Otake, Proc. Symp. Chem. Natl. Prod. 1962, 6, 136–141.
- 18C. Demetzos, K. S. Dimas, Studies in Natural Products Chemistry Vol. 25, Elsevier, 2001, pp. 235–292.
- 19R. J. Peters, Nat. Prod. Rep. 2010, 27, 1521–1530.
- 20Y. Zhao, X. Wang, Y. Zhao, Z. Yu, J. Shenyang Pharm. Univ. 2008, 25, 52–55.
- 21S. Zhang, N. Feng, J. Huang, M. Wang, L. Zhang, J. Yu, X. Dai, J. Cao, G. Huang, Bioorg. Chem. 2020, 98, 103756.
- 22L. Zhao, K.-L. Xiang, R.-X. Liu, Z.-P. Xie, S.-M. Zhang, S.-J. Dai, Bioorg. Chem. 2020, 96, 103651.
- 23K.-L. Xiang, R.-X. Liu, L. Zhao, Z.-P. Xie, S.-M. Zhang, S.-J. Dai, Phytochemistry 2020, 173, 112298.
- 24Z.-T. Deng, J.-J. Chen, C.-A. Geng, Bioorg. Chem. 2020, 95, 103571.
- 25M. Alilou, S. Marzocco, D. Hofer, S. F. Rapa, R. Asadpour, S. Schwaiger, J. Troppmair, H. Stuppner, J. Nat. Prod. 2020, 83, 2456–2468.
- 26K. J. Park, Z. Khan, L. Subedi, S. Y. Kim, K. R. Lee, J. Nat. Prod. 2020, 83, 1794–1803.
- 27T. Soumya, T. Lakshmipriya, K. D. Klika, P. Jayasree, P. Manish Kumar, Sci. Rep. 2021, 11, 1–20.
- 28J.-J. Qi, J.-S. Zhou, Y. Zhang, Y.-Y. Fan, B. Zhou, H.-C. Liu, J.-X. Zhao, J.-M. Yue, J. Nat. Prod. 2021, 84, 2971–2980.
- 29R. Jalaja, S. G. Leela, S. Mohan, M. S. Nair, R. K. Gopalan, S. B. Somappa, Bioorg. Chem. 2021, 108, 104664.
- 30Y.-Y. Chen, X.-T. Zeng, D.-Q. Xu, S.-J. Yue, R.-J. Fu, X. Yang, Z.-X. Liu, Y.-P. Tang, Phytochemistry 2022, 197, 113113.
- 31B. Cheng, F. Fang, Q. T. Zhou, Y. Li, X. W. Wu, X. R. Zhao, D. W. Bi, X. J. Zhang, R. H. Zhang, X. Ji, Chem. Biodiversity 2023, 20, e202200999.
- 32V. Sharma, T. Sharma, S. Kaul, K. K. Kapoor, M. K. Dhar, Phytochem. Rev. 2017, 16, 513–526.
- 33N. D. Idippily, Q. Zheng, C. Gan, A. Quamine, B. Su, Bioorg. Med. Chem. Lett. 2017, 27, 2292–2295.
- 34R. Jalaja, S. G. Leela, P. K. Valmiki, C. T. F. Salfeena, K. T. Ashitha, V. R. D. Krishna Rao, M. S. Nair, R. K. Gopalan, S. B. Somappa, ACS Med. Chem. Lett. 2018, 9, 662–666.
- 35S. Mazzotta, G. Carullo, A. Schiano Moriello, P. Amodeo, V. Di Marzo, M. Vega-Holm, R. M. Vitale, F. Aiello, A. Brizzi, L. De Petrocellis, Mar. Drugs 2020, 18, 519.
- 36J. de Souza-Ferrari, E. A. Silva-Junior, J. A. Vale, L. A. de Albuquerque Simoes, M. O. de Moraes-Junior, B. B. Dantas, D. A. M. de Araujo, Bioorg. Med. Chem. Lett. 2021, 52, 128393.
- 37K. S. Veena, M. S. Taniya, J. Ravindran, A. K. Thangarasu, S. Priya, R. S. Lankalapalli, Nat. Prod. Res. 2022, 36, 334–340.
- 38P. M. Szczepanik, A. A. Mikhaylov, O. Hylse, R. Kucera, P. Dadova, M. Necas, L. Kubala, K. Paruch, J. Svenda, Angew. Chem. Int. Ed. Engl. 2023, 62, e202213183.
- 39B. Wu, X.-Y. Wei, N. Wang, C. Xia, R. Bao, J. Cao, Z.-M. Zong, Z. Liu, L. Ma, G. Huang, J. Mol. Struct. 2023, 1284, 135358.
- 40Z. Liu, C. Xia, N. Wang, J. Cao, G. Huang, L. Ma, Chem. Biodiversity 2023, 20, e202300208.
- 41J. B. I. Sap, C. F. Meyer, J. Ford, N. J. W. Straathof, A. B. Dürr, M. J. Lelos, S. J. Paisey, T. A. Mollner, S. M. Hell, A. A. Trabanco, C. Genicot, C. W. am Ende, R. S. Paton, M. Tredwell, V. Gouverneur, Nature 2022, 606, 102–108.
- 42T. Araki, A. Yamamoto, M. Yamada, Histochemistry 1987, 87, 331–338.
- 43Y. Xu, D. R. Surman, L. Diggs, S. Xi, S. Gao, D. Gurusamy, K. McLoughlin, J. Drake, P. Feingold, K. Brown, Oncogene 2020, 39, 877–890.
- 44M. Fransolet, L. Noël, L. Henry, S. Labied, S. Blacher, M. Nisolle, C. Munaut, J. Assist. Reprod. Genet. 2019, 36, 349–359.
- 45C. Umbreit, J. Flanjak, C. Weiss, P. Erben, C. Aderhold, A. Faber, J. Stern-Straeter, K. Hoermann, J. D. Schultz, Anticancer Res. 2014, 34, 7061–7069.
- 46S. Revathidevi, A. K. Munirajan, Semin. Cancer Biol. 2019, 59, 80–91.
- 47Q. Xie, X. Fan, Y. Han, B.-X. Wu, B. Zhu, J. Nat. Prod. 2022, 85, 2332–2339.
- 48M. Artemenko, S. S. Zhong, S. K. To, A. S. Wong, Cancer Lett. 2022, 535, 215593.
- 49N. Hinz, M. Jücker, Cell Commun. Signaling 2019, 17, 154.
- 50M. Addie, P. Ballard, D. Buttar, C. Crafter, G. Currie, B. R. Davies, J. Debreczeni, H. Dry, P. Dudley, R. Greenwood, J. Med. Chem. 2013, 56, 2059–2073.
- 51J. Yang, P. Cron, V. M. Good, V. Thompson, B. A. Hemmings, D. Barford, Nat. Struct. Biol. 2002, 9, 940–944.
- 52A. Waterhouse, M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, R. Lepore, T. Schwede, Nucleic Acids Res. 2018, 46, W296–W303.
- 53L. Quambusch, L. Depta, I. Landel, M. Lubeck, T. Kirschner, J. Nabert, N. Uhlenbrock, J. Weisner, M. Kostka, L. M. Levy, C. Schultz-Fademrecht, F. Glanemann, K. Althoff, M. P. Müller, J. T. Siveke, D. Rauh, Nat. Commun. 2021, 12, 5297.
- 54M. K. Sharif Siam, A. Sarker, M. M. S. Sayeem, J. Biomol. Struct. Dyn. 2021, 39, 6467–6479.