Identification of a Unique Azaphilone Produced by Chaetomium globosum Isolated from Polygonatum sibiricum
Chenggang Song
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorGang Ding
Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Ministry of Education, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100193 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorGang Wu
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJian Yang
State Key Laboratory of Dao-di Herbs Breeding Base, National Resources Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, 100700 P. R. China
Search for more papers by this authorMingzhe Zhang
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorHaoyu Wang
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorDongsheng Wei
Institute of Wood Science, Department of Biology, University of Hamburg, Leuschnerstrasse 91, 21031 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Jianchun Qin
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorCorresponding Author
Lanping Guo
State Key Laboratory of Dao-di Herbs Breeding Base, National Resources Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, 100700 P. R. China
Search for more papers by this authorChenggang Song
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorGang Ding
Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Ministry of Education, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100193 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorGang Wu
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJian Yang
State Key Laboratory of Dao-di Herbs Breeding Base, National Resources Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, 100700 P. R. China
Search for more papers by this authorMingzhe Zhang
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorHaoyu Wang
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorDongsheng Wei
Institute of Wood Science, Department of Biology, University of Hamburg, Leuschnerstrasse 91, 21031 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Jianchun Qin
College of Plant Science, Jilin University, Changchun, 130062 P. R. China
Search for more papers by this authorCorresponding Author
Lanping Guo
State Key Laboratory of Dao-di Herbs Breeding Base, National Resources Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, 100700 P. R. China
Search for more papers by this authorAbstract
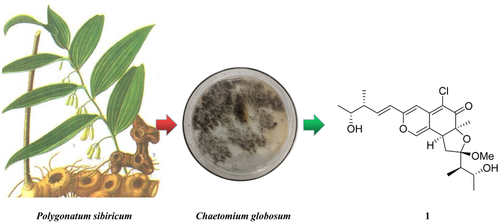
A new azaphilone, chaephilone E, eight azaphilone derivatives, and three chaetoglobosins were isolated from endophytic fungi Chaetomium globosum. The structures of the compounds were elucidated by 1D and 2D NMR as well as HR-ESI-MS data, and the absolute configuration of chaephilone E was established on the basis of electronic circular dichroism and NOESY spectrum. The activity of chaephilone E was evaluated via the cytotoxic assay (human hepatoma cell lines HepG-2) and brine shrimp (Artemia salina) bioassay.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900744-sup-0001-misc_information.pdf993.2 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. X. Wang, Z. H. Li, T. Feng, J. Li, H. Sun, R. Huang, Q. X. Yuan, H. L. Ai, J. K. Liu, ‘Curtachalasins A and B, Two Cytochalasans with a Tetracyclic Skeleton from the Endophytic Fungus Xylaria curta E10’, Org. Lett. 2018, 20, 7758–7761.
- 2O. Meghnous, L. Dehimat, P. Doumas, M. K. Laouar, F. Mosbah, O. Rached, ‘Oxidative and antioxidative responses to antimony stress by endophytic fungus Aspergillus tubingensis isolated from antimony accumulator Hedysarum pallidum Desf’, Biologia 2019, 74, 1711–1720.
- 3J. Xiao, Q. Zhang, Y. Q. Gao, J. J. Tang, A. L. Zhang, J. M. Gao, ‘Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities’, J. Agric. Food Chem. 2014, 62, 3584–3590.
- 4W. B. Han, Y. J. Zhai, Y. Gao, H. Y. Zhou, J. Xiao, G. Pescitelli, J. M. Gao, ‘Cytochalasins and an abietane-type diterpenoid with allelopathic activities from the endophytic fungus Xylaria species’, J. Agric. Food Chem. 2019, 67, 3643–3650.
- 5Q. Zhang, H. Q. Li, S. C. Zong, J. M. Gao, A. L. Zhang, ‘Chemical and bioactive diversities of the genus Chaetomium secondary metabolites’, Mini-Rev. Med. Chem. 2012, 12, 127–148.
- 6J. C. Qin, Y. M. Zhang, J. M. Gao, M. S. Bai, S. X. Yang, H. Laatsch, A. L. Zhang, ‘Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba’, Bioorg. Med. Chem. Lett. 2009, 19, 1572–1574.
- 7C. B. Tan, Z. M. Liu, S. H. Chen, ‘Oxidative Polyketones from the Mangrove-Derived Fungus Ascomycota sp. SK2YWS-L’, Sci. Rep. 2016, 6, 36609.
- 8Y. B. Liu, Y. Li, J. Qu, S. G. Ma, C. X. Zang, Y. T. Zhang, S. S. Yu, ‘Eremophilane Sesquiterpenes and Polyketones Produced by an Endophytic Guignardia Fungus from the Toxic Plant Gelsemium elegans’, J. Nat. Prod. 2015, 78, 2149–2154.
- 9J. M. Gao, S. X. Yang, J. C. Qin, ‘Azaphilones: Chemistry and Biology’, Chem. Rev. 2013, 113, 4755–4811.
- 10C. M. Chen, J. Wang, H. Zhu, J. P. Wang, Y. B. Xue, G. Z. Wei, Y. Guo, D. D. Tan, J. W. Zhang, C. P. Yin, Y. H. Zhang, ‘Chaephilones A and B, Two New Azaphilone Derivatives Isolated from Chaetomium globosum’, Chem. Biodiversity 2016, 13, 422–426.
- 11T. Yamada, M. Doi, H. Shigeta, Y. Muroga, S. Hosoe, A. Numata, R. Tanaka, ‘Absolute stereostructures of cytotoxic metabolites, chaetomugilins A–C, produced by a Chaetomium species separated from a marine fish’, Tetrahedron Lett. 2008, 49, 4192–4195.
- 12M. Yasuhide, T. Yamada, A. Numata, R. Tanaka, Chaetomugilins, ‘New Selectively Cytotoxic Metabolites, Produced by a Marine Fish-derived Chaetomium Species’, J. Antibiotica 2008, 61, 615–622.
- 13T. Yamada, Y. Muroga, M. Jinno, T. Kajimoto, Y. Usami, A. Numata, R. Tanaka, ‘New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities’, Bioorg. Med. Chem. 2011, 19, 4106–4133.
- 14T. Yamada, M. Yasuhide, H. Shigeta, A. Numata, R. Tanaka, ‘Absolute stereostructures of chaetomugilins G and H produced by a marine-fish-derived Chaetomium species’, J. Antibiotica 2009, 62, 353–357.
- 15J. V. Silverton, T. Akiyama, C. Kabuto, S. Sekita, K. Yoshihira, S. Natori, ‘X-ray analysis of chaetoglobosin A, an indol-3-yl-[13]cytochalasan from Chaetomium globosum’, Tetrahedron Lett. 1976, 17, 1349–1350.
10.1016/S0040-4039(00)78061-0 Google Scholar
- 16J. P. Springer, J. Clardy, J. M. Wells, R. J. Cole, J. W. Kirksey, R. D. Macfarlane, D. F. Torgerson, ‘Isolation and structure determination of the mycotoxin chaetoglobosin C, a new [13] cytochcalasin’, Tetrahedron Lett. 1976, 17, 1355–1356.
10.1016/S0040-4039(00)78063-4 Google Scholar
- 17G. Ding, Y. C. Song, J. R. Chen, C. Xu, H. M. Ge, X. T. Wang, R. X. Tan, ‘Chaetoglobosin U, a Cytochalasan Alkaloid from Endophytic Chaetomium globosum IFB−E019’, J. Nat. Prod. 2006, 69, 302–304.
- 18P. B. Knudsen, B. Hanna, S. Ohl, L. Sellner, T. Zenz, H. Döhner, S. Stilgenbauer, T. Larsen, P. Lichter, M. Seiffert, ‘Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton’, Leukemia 2014, 28, 1289–1298.
- 19H. M. Ge, W. Y. Zhang, G. Ding, P. Saparpakorn, Y. C. Song, S. Hannongbua, R. X. Tan, ‘Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture’, Chem. Commun. 2008, 59, 78–5980.
- 20X. Li, Y. Tian, S. X. Yang, Y. M. Zhang, J. C. Qin, ‘Cytotoxic azaphilone alkaloids from Chaetomium globosum TY1’, Bioorg. Med. Chem. Lett. 2013, 23, 2945–2947.