Chemical Characterization and Phytotoxic Effects of the Aerial Parts of Ruzigrass (Urochloa ruziziensis)
Beatriz Pereira Moreno
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorGislaine Cristiane Mantovanelli
Departamento de Bioquímica, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorLetycia Lopes Ricardo
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorAdriano Antônio Silva
Departamento de Ciências da Natureza, Universidade Federal do Acre, BR-364 KM 04, 69915-900 Rio Branco, Acre, Brazil
Search for more papers by this authorRubem Silvério De Oliveira Jr.
Departamento de Agronomia, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorEmy Luiza Ishii-Iwamoto
Departamento de Bioquímica, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorMaria Helena Sarragiotto
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorCorresponding Author
Debora Cristina Baldoqui
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorBeatriz Pereira Moreno
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorGislaine Cristiane Mantovanelli
Departamento de Bioquímica, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorLetycia Lopes Ricardo
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorAdriano Antônio Silva
Departamento de Ciências da Natureza, Universidade Federal do Acre, BR-364 KM 04, 69915-900 Rio Branco, Acre, Brazil
Search for more papers by this authorRubem Silvério De Oliveira Jr.
Departamento de Agronomia, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorEmy Luiza Ishii-Iwamoto
Departamento de Bioquímica, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorMaria Helena Sarragiotto
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorCorresponding Author
Debora Cristina Baldoqui
Departamento de Química, Universidade Estadual de Maringá, Av. Colombo, 5.790, 87020-900 Maringá, Paraná, Brazil
Search for more papers by this authorAbstract
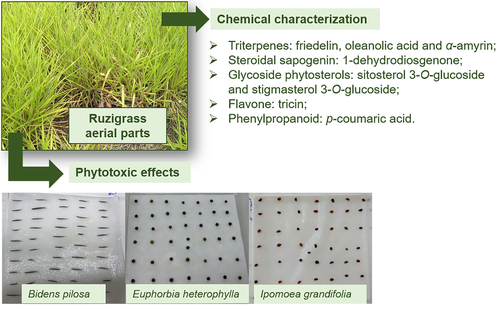
Studies of the phytotoxic effects between plants can be a crucial tool in the discovery of innovative compounds with herbicide potential. In this sense, we can highlight ruzigrass (Urochloa ruziziensis), which is traditionally used in the crop rotation system in order to reduce weed emergence. The aim of this work was to characterize the secondary metabolites of ruzigrass and to evaluate its phytotoxic effects. In total, eight compounds were isolated: friedelin, oleanolic acid, α-amyrin, 1-dehydrodiosgenone, sitosterol and stigmasterol glycosides, tricin and p-coumaric acid. Phytotoxic effects of the crude methanolic extract and fractions of ruzigrass were assessed using germination rate, initial seedling growth, and biomass of Bidens pilosa, Euphorbia heterophylla and Ipomoea grandifolia. Chemometric analysis discriminated the weed species into three groups, and B. pilosa was the most affected by fractions of ruzigrass. The phytotoxic activities of 1-dehydrodiosgenone, tricin, and p-coumaric acid are also reported, and p-coumaric acid and 1-dehydrodiosgenone were active against B. pilosa.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900694-sup-0001-misc_information.pdf248.4 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. B. Florindo, N. R. Silva, L. M. Romualdo, F. F. Silva, P. H. C. Luz, V. R. Herling, O. M. Bruno, ‘Brachiaria species identification using imaging techniques based on fractal descriptors’, Comp. Electron. Agric. 2014, 103, 48–54.
- 2P. Wenzl, A. L. Chaves, J. E. Mayer, I. M. Rao, M. G. Nair, ‘Roots of nutrient deprived Brachiaria species accumulate 1,3-di-O-trans-feruloylquinic acid’, Phytochemistry 2000, 55, 389–395.
- 3C. Arroyave, J. Barceló, C. Poschenrieder, R. Tolrà, ‘Aluminium-induced changes in root epidermal cell patterning, a distinctive feature of hyperresistance to Al in Brachiaria decumbens’, J. Inorg. Biochem. 2011, 105, 1477–1483.
- 4T. C. Faccin, F. Riet-Correa, F. S. Rodrigues, A. C. Santos, G. K. A. Melo, J. A. Silva, R. Ferreira, C. C. B. F. Ítavo, R. A. A. Lemos, ‘Poisoning by Brachiaria brizantha in flocks of naïve and experienced sheep’, Toxicon 2014, 82, 1–8.
- 5M. P. Nepomuceno, R. M. Varela, P. L. C. A. Alves, J. V. F. Martins, ‘Períodos de dessecação de Urochloa ruziziensis e seu reflexo na produtividade da soja RR’, Planta Daninha 2012, 30, 557–565.
- 6K. B. Brum, M. Haraguchi, M. B. Garutti, F. N. Nóbrega, B. Rosa, M. C. S. Fioravanti, ‘Steroidal saponin concentrations in Brachiaria decumbens and B. brizantha at different developmental stages’, Cienc. Rural 2009, 39, 279–281.
- 7M. Nepomuceno, N. Chinchilla, R. M. Varela, J. M. G. Molinillo, R. Lacret, P. L. C. A. Alves, F. A. Macias, ‘Chemical evidence for the effect of Urochloa ruziziensis on glyphosate-resistant soybeans’, Pest Manage. Sci. 2017, 73, 2071–2078.
- 8G. Keller-Grein, B. L. Maass, J. Hanson, in ‘Brachiaria: Biology, Agronomy, and Improvement’, Eds. J. W. Miles, B. L. Maass, C. B. do Valle, V. Kumble, CIAT and EMBRAPA; CIAT Publication No. 259, Columbia, 1996, p. 16–42.
- 9D. S. Almeida, C. A. Rosolem, ‘Ruzigrass grown in rotation with soybean increases soil labile phosphorus’, Agron. J. 2016, 108, 1–9.
- 10A. Merlin, C. A. Rosolem, Z. L. He, ‘Non-labile phosphorus acquisition by Brachiarias’, J. Plant Nutr. 2016, 39, 1319–1327.
- 11M. C. M. Macedo, ‘Integração lavoura e pecuária: o estado da arte e inovações tecnológicas’, R. Bras. Zootec. 2009, 38, 133–146.
- 12R. S. Oliveira Jr., F. A. Rios, J. Constantin, E. L. Ishii-Iwamoto, A. Gemelli, P. E. Martini, ‘Grass straw mulching to suppress emergence and early growth of weeds’, Planta Daninha 2014, 32, 11–17.
- 13J. R. Teasdale, C. B. Coffman, R. W. Mangum, ‘Potential long-term benefits of no-tillage and organic cropping systems for grain production and soil improvement’, Agron. J. 2007, 99, 1297–1305.
- 14E. Borghi, N. V. Costa, C. A. C. Crusciol, G. P. Mateus, ‘Influence of the spatial distribution of maize and Brachiaria brizantha intercropping on the weed population under no-tillage’, Planta Daninha 2008, 26, 559–68.
- 15F. R. Echer, G. S. A. Castro, J. C. Bogiani, C. A. Rosolem, ‘Initial growth and nutrient absorption of cotton cultivated on Congo grass residues’, Planta Daninha 2012, 30, 783–790.
- 16C. A. Chioderoli, L. M. M. Mello, P. J. Grigolli, J. O. R. Silva, A. L. Cesarin, ‘Consorciação de braquiárias com milho outonal em plantio direto sob pivô central’, Eng. Agric. 2010, 30, 1101–1109.
- 17J. R. Teasdale, C. L. Mohler, ‘The quantitative relationship between weed emergence and the physical properties of mulches’, Weed Sci. 2000, 48, 385–392.
- 18L. L. Ricardo, D. I. Bernardi, G. C. Mantovanelli, B. P. Moreno, M. S. Mito, A. A. Silva, R. S. Oliveira Jr., E. L. Ishii-Iwamoto, M. H. Sarragiotto, D. C. Baldoqui, ‘Phytochemical investigation and phytotoxic activity of aerial parts of oilseed radish (Raphanus sativus var. oleifer Stokes)’, Biochem. Syst. Ecol. 2018, 78, 52–58.
- 19E. M. Pergo, D. Abrahim, P. C. S. Silva, K. A. Kern, L. J. Silva, E. Voll, E. L. Ishii-Iwamoto, ‘Bidens pilosa L. exhibits high sensitivity to coumarin in comparison with three other weed species’, J. Chem. Ecol. 2008, 34, 499–507.
- 20M. M. Trezzi, R. A. Vidal, D. Mattei, H. L. Silva, C. E. Carnieleto, M. S. Gustmann, R. Viola, A. Machado, ‘Efeitos de resíduos na parte aérea de sorgo, milho e aveia na emergência e no desenvolvimento de plântulas de leiteiro (Euphorbia heterophylla) resistentes a inibidores da ALS’, Planta Daninha 2006, 24, 443–450.
10.1590/S0100-83582006000300004 Google Scholar
- 21K. Kobayashi, ‘Factors affecting phytotoxic activity of allelochemicals in soil’, Weed Biol. Manage. 2004, 4, 1–7.
- 22H. Sodaeizadeh, M. Rafieiolhossaini, J. Havlík, P. Van Damme, ‘Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances’, Plant Growth Regul. 2009, 59, 227–236.
- 23J. R. Quasem, N. N. Issa, ‘Germination and growth management of some common annual weeds by phytotoxicity of selected vegetable crops’, Sci. Hortic. 2018, 233, 431–445.
- 24A. Synowiec, D. Kalemba, E. Drozdek, J. Bocianowski, ‘Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops’, J. Pestic. Sci. 2017, 90, 407–419.
- 25A. P. S. Souza Filho, S. M. Alves, F. J. C. Figueiredo, ‘Efeitos alelopáticos do calopogônio em função de sua idade e da densidade de sementes da planta receptora’, Planta Daninha 2003, 21, 211–218.
10.1590/S0100-83582003000200006 Google Scholar
- 26H. M. Kruidhof, E. R. Gallandt, E. R. Haramoto, L. Bastiaans, ‘Selective weed suppression by cover crop residues: effects of seed mass and timing of species’ sensitivity’, Weed Res. 2011, 51, 177–186.
- 27F. E. Dayan, ‘Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor’, Planta 2006, 224, 339–346.
- 28K. M. G. G. Jayasuriya, J. M. Baskin, R. L. Geneve, C. C. Baskin, ‘Morphology and anatomy of physical dormancy in Ipomoea lacunosa: identification of the water gap in seeds of Convolvulaceae (Solanales)’, Ann. Bot. 2007, 100, 13–22.
- 29M. E. Hanley, M. D. Whiting, ‘Insecticides and arable weeds: Effects on germination and seedling growth’, Ecotoxicology 2005, 14, 483–490.
- 30A. Gniazdowska, R. Bogatek, ‘Allelopathic interactions between plants. Multi-site action of allelochemicals’, Acta Physiol. Plant. 2005, 27, 395–407.
- 31M. A. Alam, M. A. Hakim, A. S. Juraimi, M. Y. Rafii, M. M. Hasan, F. Aslani, ‘Potential allelopathic effects of rice plant aqueous extracts on germination and seedling growth of some rice field common weeds’, Ital. J. Agron. 2018, 13, 134–140.
- 32C. O. Martendal, G. C. Mantovanelli, B. Reis, C. Cavaleiro, E. L. I. Iwamoto, C. M. Bonato, ‘Effects of Ocimum gratissimum L. extract on the germination, respiration and growth of Euphorbia heterophylla L’, Allelopathy J. 2018, 44, 29–44.
- 33M. F. O. Almeida, A. C. R. Melo, M. L. B. Pinheiro, J. R. A. Silva, A. D. L. Souza, A. Barison, F. R. Campos, A. C. F. Amaral, G. M. C. Machado, L. L. P. Leon, ‘Constituintes químicos e atividade leishmanicida de Gustavia elliptica (Lecythidaceae)’, Quim. Nova 2014, 34, 1182–1187.
10.1590/S0100-40422011000700015 Google Scholar
- 34Z. Güvenalp, H. Özbek, A. Kuruüzüm-Uz, C. Kazaz, L. Ö. Demirezer, ‘Secondary metabolites from Nepeta heliotropifolia’, Turk. J. Chem. 2009, 33, 667–675.
- 35M. O. Dias, L. Hamerski, A. C. Pinto, ‘Separação semipreparativa de α e β-amirina por cromatografia líquida de alta eficiência’, Quim. Nova 2011, 34, 704–706.
- 36X. F. Huang, Y. Y. Lin, L. Y. Kong, ‘Steroids from the roots of Asparagus officinalis and their cytotoxic activity’, J. Integr. Plant Biol. 2008, 50, 717–722.
- 37H. Kojima, N. Sato, A. Hatano, H. Ogura, ‘Sterol glucosides from Prunella vulgaris’, Phytochemistry 1990, 29, 2351–2355.
- 38N. Bai, K. He, M. Roller, C. S. Lai, L. Bai, M. H. Pan, ‘Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines’, J. Agric. Food Chem. 2015, 63, 2458–2463.
- 39A. P. S. Souza Filho, A. A. G. Pereira, J. C. Bayma, ‘Aleloquímico produzido pela gramínea forrageira Brachiaria humidicola’, Planta Daninha 2005, 23, 25–32.
10.1590/S0100-83582005000100004 Google Scholar
- 40C. Renard, ‘Présence de composés phénoliques dans les envelopes des épillets de Brachiaria ruziziensis Germain et Evrard’, Bull. Soc. R. Bot. Belg. 1976, 109, 227–230.
- 41L. S. Santos, J. C. L. Santos, A. P. S. Souza Filho, M. J. C. Corrêa, T. A. M. Veiga, V. C. M. Freitas, I. C. S. Ferreira, N. S. Gonçalves, C. E. Silva, G. M. S. P. Guilhon, ‘Atividade alelopática de substâncias químicas isoladas do Capim-marandu e suas variações em função do pH’, Planta Daninha 2008, 26, 531–538.
- 42A. González-Coloma, C. López-Balboa, O. Santana, M. Reina, B. M. Fraga, ‘Triterpene-based plant defenses’, Phytochem. Rev. 2011, 10, 245–260.
- 43R. R. Trevisan, C. P. Lima, C. M. S. Miyazaki, F. A. Pesci, C. B. Silva, B. C. K. Hirota, A. L. L. Lordello, O. G. Miguel, M. D. Miguel, S. M. W. Zanin, ‘Avaliação da atividade fitotóxica com enfoque alelopático do extrato das cascas de Celtis iguanaea (Jacq.) Sargent Ulmaceae e purificação de dois triterpenos’, Rev. Bras. Plant. Med. 2012, 14, 494–499.
- 44D. S. S. Kpoviessi, G. C. Accrombessi, J. D. Gbénou, F. A. Gbaguidi, D. K. Kossou, M. Moudachirou, J. J. Quentin-Leclercq, ‘Cytotoxic activities of sterols and triterpenes identified by GC/MS in Justicia anselliana (NEES) T. Anders active fractions and allelopathic effects on cowpea (Vigna unguiculata (L.) Walp) plant’, J. Soc. Ouest-Afr. Chim. 2008, 26, 59–67.
- 45A. L. Anaya, R. Mata, J. J. Sims, A. González-Coloma, R. Cruz-Ortega, A. Guadaño, B. E. Hernández-Bautista, S. L. Midland, G. Ríos, A. Gómez-Pompa, ‘Allelochemical potential of Callicarpa acuminata’, J. Chem. Ecol. 2003, 29, 2761–2776.
- 46M. H. Inoue, D. C. Santana, K. S. S. Vilhena, A. P. S. Souza Filho, G. M. S. P. Guilhon, A. C. S. Possamai, L. E. Silva, R. Dallacort, ‘Avaliação do potencial alelopático de substâncias isoladas em sementes de araticum (Annona crassiflora)’, Planta Daninha 2010, 28, 735–741.
- 47F. A. Macías, J. M. G. Molinillo, R. M. Varela, J. C. G. Galindo, ‘Allelopathy - a natural alternative for weed control’, Pest Manage. Sci. 2007, 63, 327–348.
- 48C. Kong, W. Liang, X. Xu, F. Hu, P. Wang, Y. Jiang, ‘Release and activity of allelochemicals from allelopathic rice seedlings’, J. Agric. Food Chem. 2004, 52, 2861–2865.
- 49L. A. Weston, B. A. Burke, A. R. Putnam, ‘Isolation, characterization and activity of phytotoxic compounds from quackgrass [Agropyron repens (L.) Beauv.]’, J. Chem. Ecol. 1987, 13, 403–421.
- 50I. Heap, ‘International survey of herbicide resistant weeds’, Available: www.weedscience.org. Accessed on 03 November 2018.
- 51F. Araniti, M. Marrelli, A. Lupini, F. Mercati, G. A. Statti, M. R. Abenavoli, ‘Phytotoxic activity of Cachrys pungens Jan, a Mediterranean species: separation, identification and quantification of potential allelochemicals’, Acta Physiol. Plant. 2014, 36, 1071–1083.
- 52Y. Luo, J. Liang, G. Zeng, M. Chen, D. Mo, G. Li, D. Zhang, ‘Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects’, Waste Manage. 2018, 71, 109–114.