BF12, a Novel Benzofuran, Exhibits Antitumor Activity by Inhibiting Microtubules and the PI3K/Akt/mTOR Signaling Pathway in Human Cervical Cancer Cells
Yiting Gao
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorCorresponding Author
Cheng Ma
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorXuezhao Feng
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorYang Liu
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorXiaohelaiti Haimiti
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorYiting Gao
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorCorresponding Author
Cheng Ma
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorXuezhao Feng
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorYang Liu
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorXiaohelaiti Haimiti
Department of Medicinal and Organic Chemistry, School of Pharmacy, Xinjiang Medical University, Beijing Road 393#, Xinshi District, Urumqi, 830011 P. R. China
Search for more papers by this authorAbstract
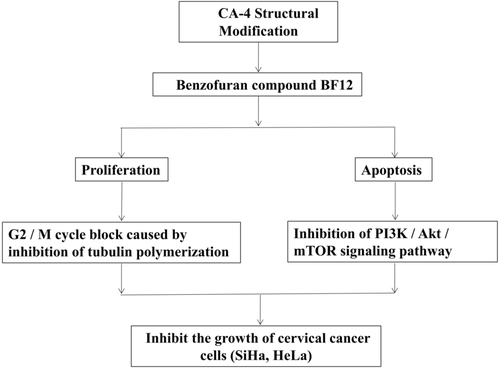
BF12 [(2E)-3-[6-Methoxy-2-(3,4,5-trimethoxybenzoyl)-1-benzofuran-5-yl]prop-2-enoic acid], a novel derivative of combretastatin A-4 (CA-4), was previously found to inhibit tumor cell lines, with a particularly strong inhibitory effect on cervical cancer cells. In this study, we investigated the microtubule polymerization effects and apoptosis signaling mechanism of BF12. BF12 showed a potent efficiency against cervical cancer cells, SiHa and HeLa, with IC50 values of 1.10 and 1.06 μm, respectively. The cellular mechanism studies revealed that BF12 induced G2/M phase arrest and apoptosis in SiHa and HeLa cells, which were associated with alterations in the expression of the cell G2/M cycle checkpoint-related proteins (cyclin B1 and cdc2) and alterations in the levels of apoptosis-related proteins (P53, caspase-3, Bcl-2, and Bax) of these cells, respectively. Western blot analysis showed that BF12 inhibited the PI3 K/Akt/mTOR signaling pathway and induced apoptosis in human cervical cancer cells. BF12 was identified as a tubulin polymerization inhibitor, evidenced by the effective inhibition of tubulin polymerization and heavily disrupted microtubule networks in living SiHa and HeLa cells. By inhibiting the PI3 K/Akt/mTOR signaling pathway and inducing apoptosis in human cervical cancer cells, BF12 shows promise for use as a microtubule inhibitor.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900622-sup-0001-misc_information.pdf226.4 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent, A. Jemal, ‘Global cancer statistics, 2012’, Ca-Cancer J. Clin. 2015, 65, 87–108.
- 2Y. Li, N. Cui, P. S. Zheng, W. T. Yang, ‘BMX/Etk promotes cell proliferation and tumorigenicity of cervical cancer cells through PI3 K/AKT/mTOR and STAT3 pathways’, Oncotarget 2017, 8, 49238–49252.
- 3A. Li, Y. Gu, X. Li, H. Sun, H. Zha, J. Xie, J. Zhao, M. Huang, L. Chen, Q. Peng, Y. Zhang, Y. Weng, L. Zhou, ‘S100A6 promotes the proliferation and migration of cervical cancer cells via the PI3 K/Akt signaling pathway’, Oncol. Lett. 2018, 15, 5685–5693.
- 4N. Husseinzadeh, H. D. Husseinzadeh, ‘mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review’, Gynecol. Oncol. 2014, 133, 375–381.
- 5Y. Cheng, J. P. Liou, W. Y. Lai, C. Y. Chang, W. Y. Pan, C. C. Kuo, J. Y. Chang, ‘Abstract 4461: MPT0B098, A novel microtubule inhibitor, displays potent anti-angiogenic activity via destabilizing hypoxia-inducible factor-1alpha mRNA’, Cancer Res. 2011, 71, 4461.
- 6Y. Fujiwara, Y. Hosokawa, K. Watanabe, S. Tanimura, K. Ozaki, M. Kohno, ‘Blockade of the phosphatidylinositol-3-kinase-Akt signaling pathway enhances the induction of apoptosis by microtubule-destabilizing agents in tumor cells in which the pathway is constitutively activated’, Mol. Cancer Ther. 2007, 6, 1133–1142.
- 7J. A. Woods, J. A. Hadfield, G. R. Pettit, B. W. Fox, A. T. Mcgown, ‘The interaction with tubulin of a series of stilbenes based on combretastatin A-4’, Br. J. Cancer. 1995, 71, 705–711.
- 8G. R. Pettit, S. B. Singh, E. Hamel, C. M. Lin, D. S. Alberts, D. Garcia-Kendal, ‘Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4’, Experientia 1989, 45, 209–211.
- 9F. Ye, L. Yang, C. Ma, ‘Synthesis and cytotoxic evaluation of combretastatin A-4 analogs of benzo[b]furans’, Mon. Chem. 2017, 148, 1823–1832.
- 10K. Aleksandrzak, A. T. McGown, J. A. Hadfield, ‘Antimitotic activity of diaryl compounds with structural features resembling combretastatin A-4’, Anti-Cancer Drugs 1998, 9, 545–550.
- 11S. Sur, D. K. Agrawal, ‘Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies’, Mol. Cell. Biochem. 2016, 416, 33–46.
- 12R. W. Deibler, M. W. Kirschner, ‘Quantitative Reconstitution of Mitotic CDK1 Activation in Somatic Cell Extracts’, Mol. Cell 2010, 37, 753–767.
- 13D. V. Bulavin, Y. Higashimoto, Z. N. Demidenko, S. Meek, P. Graves, C. Phillips, H. Zhao, S. A. Moody, E. Appella, H. Piwnica-Worms, A. J. Fornace Jr., ‘Dual phosphorylation controls Cdc25 phosphatases and mitotic entry’, Nat. Cell Biol. 2003, 5, 545–551.
- 14S. E. Zheng, S. Xiong, F. Lin, G.-L. Qiao, T. Feng, Z. Shen, D.-L. Min, C.-L. Zhang, Y. Yao, ‘Pirarubicin inhibits multidrug-resistant osteosarcoma cell proliferation through induction of G2/M phase cell cycle arrest’, Acta Pharmacol. Sin. 2012, 33, 832–838.
- 15H. Loffler, B. Rebacz, A. D. Ho, J. Lukas, J. Bartek, A. Kramer, ‘Chk1-Dependent Regulation of Cdc25B Functions to Coordinate Mitotic Events’, Cell Cycle 2006, 5, 2543–2547.
- 16P. H. Lu, F.-L. Kung, S.-C. Kuo, S.-C. Chueh, J.-H. Guh, ‘Investigation of anti-tumor mechanisms of K2154: characterization of tubulin isotypes, mitotic arrest and apoptotic machinery’, Naunyn-Schmiedeberg′s Arch. Pharmacol. 2006, 374, 223–233.
- 17A. Vuletic, G. Konjevic, D. Milanovic, S. Ruzdijic, V. Jurisic, ‘Antiproliferative Effect of 13-cis-Retinoic Acid is Associated with Granulocyte Differentiation and Decrease in Cyclin B1 and Bcl-2 Protein Levels in G0/G1 Arrested HL-60 Cells’, Pathol. Oncol. Res. 2010, 16, 393–401.
- 18C. Bürger, M. Wick, R. Müller, ‘Lineage-specific regulation of cell-cycle gene expression in differentiating myeloid cells’, J. Cell Sci. 1994, 107, 2047–2054.
- 19L.-C. Chang, Y.-L. Yu, C.-Y. Liu, Y.-Y. Cheng, R.-H. Chou, M.-T. Hsieh, H.-Y. Lin, H.-Y. Hung, L.-J. Huang, Y.-C. Wu, S.-C. Kuo, ‘The newly synthesized 2-arylnaphthyridin-4-one, CSC-3436, induces apoptosis of non-small cell lung cancer cells by inhibiting tubulin dynamics and activating CDK1’, Cancer Chemother. Pharmacol. 2015, 75, 1303–1315.
- 20R. Romagnoli, P. G. Baraldi, M. D. Carrion, C. L. Cara, O. Cruz-Lopez, M. Tolomeo, S. Grimaudo, A. D. Cristina, M. R. Pipitone, J. Balzarini, ‘Design, synthesis and structure-activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of inhibitors of tubulin polymerization’, Bioorg. Med. Chem. 2009, 17, 6862–6871.
- 21B. Geiger, S. J. Singer, ‘Association of Microtubules and Intermediate Filaments in Chicken Gizzard Cells as Detected by Double Immunofluorescence’, Proc. Natl. Acad. Sci. USA 1980, 77, 4769–4773.
- 22S. Honore, K. Kamath, D. Braguer, L. Wilson, C. Briand, M. A. Jordan, ‘Suppression of microtubule dynamics by discodermolide by a novel mechanism is associated with mitotic arrest and inhibition of tumor cell proliferation’, Mol. Cancer Ther. 2003, 2, 1303–1311.
- 23N. Lampiasi, D. Foderà, N. D′Alessandro, A. Cusimano, A. Azzolina, C. Tripodo, A. M. Florena, M. I. Minervini, M. Notarbartolo, G. Montalto, ‘The selective cyclooxygenase-1 inhibitor SC-560 suppresses cell proliferation and induces apoptosis in human hepatocellular carcinoma cells’, Int. J. Mol. Med. 2006, 17, 245–252.
- 24G. Lizard, S. Fournel, L. Genestier, N. Dhedin, C. Chaput, M. Flacher, M. Mutin, G. Panaye, J. P. Revillard, ‘Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis’, Cytometry Part A 2010, 21, 275–283.
- 25M. S. Lin, W. C. Chen, X. Bai, Y. D. Wang, ‘Activation of peroxisome proliferator-activated receptor gamma inhibits cell growth via apoptosis and arrest of the cell cycle in human colorectal cancer’, J. Dig. Dis. 2010, 8, 82–88.
- 26T. F. Franke, C. P. Hornik, L. Segev, G. A. Shostak, C. Sugimoto, ‘PI3 K/Akt and apoptosis: size matters’, Oncogene 2003, 22, 8983–8998.
- 27V. Serra, B. Markman, M. Scaltriti, P. J. A. Eichhorn, J. Baselga, ‘NVP-BEZ235, dual PI3 K/mTOR inhibitor, prevents PI3 K signaling and inhibits the growth of cancer cells with activating PI3 K mutations’, Cancer Res. 2008, 68, 8022–8030.
- 28X. Xu, O. K. Kwon, I. S. Shin, J. R. Mali, D. S. Harmalkar, ‘Novel benzofuran derivative DK-1014 attenuates lung inflammation via blocking of MAPK/AP-1 and AKT/mTOR signaling in vitro and in vivo’, Sci. Rep. 2019, 9, 862–875.
- 29H. Z. Hu, Y. B. Yang, X. D. Xu, H. W. Shen, Y. M. Shu, Z. Ren, X. M. Li, H. M. Shen, H. T. Zeng, ‘Oridonin induces apoptosis via PI3 K/Akt pathway in cervical carcinoma HeLa cell line’, Acta Pharmacol. Sin. 2010, 28, 1819–1826.
- 30S. B. Prasad, S. S. Yadav, M. Das, A. Modi, S. Kumari, L. K. Pandey, S. Singh, S. Pradhan, G. Narayan, ‘PI3 K/AKT pathway-mediated regulation of p27(Kip1) is associated with cell cycle arrest and apoptosis in cervical cancer’, Cell. Oncol. 2015, 38, 215–225.
- 31A. Kamal, V. Lakshma Nayak, N. Nagesh, M. V. P. S. Vishnuvardhan, N. V. Subba Reddy, ‘Benzo[b]furan derivatives induces apoptosis by targeting the PI3 K/Akt/mTOR signaling pathway in human breast cancer cells’, Bioorg. Chem. 2016, 66, 124–131.
- 32M. Hidalgo, E. K. Rowinsky, ‘The rapamycin-sensitive signal transduction pathway as a target for cancer therapy’, Oncogene 2000, 19, 6680–6686.
- 33T. Bohnacker, A. E. Prota, F. Beaufils, J. E. Burke, A. Melone, A. J. Inglis, D. Rageot, A. M. Sele, V. Cmiljanovic, N. Cmiljanovic, ‘Deconvolution of Buparlisib's mechanism of action defines specific PI3 K and tubulin inhibitors for therapeutic intervention’, Nat. Commun. 2017, 8, 14683–14696.
- 34Z. Chen, J. Li, X. Song, Z. Wang, W. Yue, ‘Use of a novel sonosensitizer in sonodynamic therapy of U251 glioma cells in vitro’, Exp. Ther. Med. 2012, 3, 273–278.
- 35S. Kasibhatla, G. P. Amarantemendes, D. Finucane, T. Brunner, E. Bossywetzel, D. R. Green, ‘Staining of suspension cells with Hoechst 33258 to detect apoptosis’, Cold Spring Harbor Protocols 2006, 21, 659–667.
- 36T. Hayano, M. Garg, D. Yin, M. Sudo, N. Kawamata, S. Shi, W. Chien, L. W. Ding, G. Leong, S. Mori, ‘SOX7 is down-regulated in lung cancer’, J. Exp. Clin. Cancer Res. 2013, 32, 17–28.
- 37O. S. Frankfurt, A. Krishan, ‘Enzyme-Linked Immunosorbent Assay (ELISA) for the Specific Detection of Apoptotic Cells and Its Application to Rapid Drug Screening’, J. Immunol. Methods 2001, 253, 133–144.
- 38G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell, A. J. Olson, ‘AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility’, J. Comput. Chem. 2010, 30, 2785–2791.
- 39H. M. Berman, M. J. Gabanyi, C. R. Groom, J. E. Johnson, G. N. Murshudov, R. A. Nicholls, V. Reddy, T. Schwede, M. D. Zimmerman, J. Westbrook, ‘Data to knowledge: How to get meaning from your result’, IUCrJ 2015, 2, 45–58.
- 40Y. F. Yao, Z. C. Wang, S. Y. Wu, Q. F. Li, C. Yu, X. Y. Liang, P. C. Lv, Y. T. Duan, H. L. Zhu, ‘Identification of novel 1-indolyl acetate-5-nitroimidazole derivatives of combretastatin A-4 as potential tubulin polymerization inhibitors’, Biochem. Pharmacol. 2017, 137, 10–28.
- 41L. O′Donnell, M. K. O′Bryan, ‘Microtubules and spermatogenesis’, Semin. Cell Dev. Biol. 2014, 30, 45–54.
- 42P. M. N. Kischel, H. Priebe, W. Splettstösser, E. J. Finke, R. Grunow, ‘Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia’, Clin. Vaccine Immunol. 2004, 11, 1008–1015.
10.1128/CDLI.11.6.1008-1015.2004 Google Scholar
- 43C. W. Lewis, R. G. Taylor, P. M. Kubara, K. Marshall, L. Meijer, R. M. Golsteyn, ‘A Western blot assay to measure cyclin dependent kinase activity in cells or in vitro without the use of radioisotopes’, FEBS Lett. 2013, 587, 3089–3095.