Crosstalk between ferroptosis and stress—Implications in cancer therapeutic responses
Cheng Zhang and Jiao-jiao Yu contributed equally to this study.
Abstract
Ferroptosis is a newly discovered form of cell death that is characterized by the accumulation of iron-dependent lipid peroxidation. Research on ferroptosis has seen exponential growth over the past few years. Tumor cells are strongly dependent on iron for their growth, which makes them develop mechanisms to increase iron uptake and inhibit iron output, thereby completing iron accumulation. Ferroptosis can be induced or inhibited by various stresses through multiple mechanisms, making it stands at the crossroads of stresses related cancer cell fate determination. In this review, we give a brief summary of ferroptosis hallmarks and provide a systematic analysis of the current molecular mechanisms and regulatory networks of diverse stress conditions on ferroptosis. We also discuss the relationships between ferroptosis and cancer therapy responses to further understand potential targets and therapeutic strategies for cancer treatment.
Abbreviations
-
- AA
-
- amino acid
-
- AA
-
- arachidonic acid
-
- ACC
-
- acetyl-CoA carboxylase
-
- Acetyl-CoA
-
- acetyl coenzyme A
-
- ACSL3
-
- acyl-CoA synthetase long chain family member 3
-
- ACSL4
-
- acyl-CoA synthetase long-chain family member 4
-
- AdA
-
- adrenaline
-
- ALOX15
-
- arachidonate lipoxygenase 15
-
- ALOXE3
-
- arachidonate lipoxygenase 3
-
- AMP
-
- adenosine monophosphate
-
- AMPK
-
- AMP-activated protein kinase
-
- APC
-
- antigen-presenting cell
-
- AR
-
- androgen receptor
-
- ARE
-
- antioxidant response element
-
- ARNTL
-
- aryl hydrocarbon receptor nuclear translocator-like protein 1
-
- ASK1
-
- apoptosis signal-regulating kinase 1
-
- ATF4
-
- activating transcription factor 4
-
- ATM
-
- Ataxia-telangiectasia mutated
-
- ATP
-
- adenosine triphosphate
-
- ATR
-
- ATM- and Rad3-Related
-
- AUR
-
- auranofin
-
- BAK
-
- Bcl-2 homologous antagonist/killer
-
- BAP1
-
- BRCA1-associated protein 1
-
- BAX
-
- Bcl-2-associated X
-
- BECN1
-
- Beclin 1
-
- BSO
-
- buthionine sulfoximine
-
- CA9
-
- carbonic anhydrase 9
-
- CAF
-
- cancer-associated fibroblast
-
- CCC
-
- clear-cell carcinoma
-
- CDKN1A
-
- cyclin-dependent kinase inhibitor 1A
-
- CDO1
-
- cysteine dioxygenase type 1
-
- CHAC1
-
- ChaC glutathione-specific gamma-glutamylcyclotransferase 1
-
- CoQ
-
- coenzyme Q10
-
- CoQH2
-
- reduced form of coenzyme Q10
-
- CR
-
- complete response
-
- CTH
-
- cystathionine gamma-lyase
-
- DAMP
-
- damage-associated molecular pattern
-
- DDR
-
- DNA Damage Response
-
- DFX
-
- deferoxamine
-
- DGLA
-
- dihomo-gamma-linolenic acid
-
- DHODH
-
- dihydroorotate dehydrogenase
-
- DMF
-
- dimethyl fumarate
-
- DMT1
-
- divalent metal transporter 1
-
- DOX
-
- doxorubicin
-
- DPP4
-
- dipeptidyl peptidase 4
-
- DSB
-
- double-strand break
-
- EGFR
-
- epidermal growth factor receptor
-
- EGLN2
-
- egl nine homolog 2
-
- eIF2α
-
- eukaryotic initiation factor 2α
-
- EMT
-
- epithelial–mesenchymal transition
-
- ER
-
- endoplasmic reticulum
-
- ERK
-
- extracellular signal-regulated kinase
-
- FBXL2
-
- F-box/LRR-repeat protein 2
-
- FIN
-
- ferroptosis inducer
-
- FPN1
-
- ferroportin
-
- FSP1
-
- ferroptosis suppressor protein 1
-
- FTH
-
- ferritin heavy chain
-
- FTL
-
- ferritin light chain
-
- FXN
-
- frataxin
-
- GBM
-
- glioblastoma multiforme
-
- GC
-
- gastric cancer
-
- GCLC
-
- glutamate-cysteine ligase catalytic subunit
-
- GLS2
-
- glutaminase 2
-
- GPX4
-
- glutathione peroxidase 4
-
- GSH
-
- glutathione
-
- GSSG
-
- oxidized glutathione
-
- H2Aub
-
- H2A ubiquitination
-
- HCAR1
-
- hydroxycarboxylic acid receptor 1
-
- HCC
-
- hepatocellular carcinoma
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HIF
-
- hypoxia-inducible factor
-
- HIF-1α
-
- hypoxia-inducible factor 1 subunit α
-
- HIF-2α
-
- hypoxia-inducible factor 2 subunit α
-
- HILPDA
-
- hypoxia-inducible, lipid droplet-associated protein
-
- HMGB1
-
- high mobility group box 1
-
- HMG-CoA
-
- 3-hydroxy-3-methylglutaryl coenzyme A
-
- hnRNPA1
-
- heterogeneous nuclear ribonucleoprotein A1
-
- HO•
-
- hydroxyl
-
- HPSCC
-
- hypopharyngeal squamous carcinoma
-
- HSF
-
- heat shock factor
-
- HSF1
-
- heat shock factor 1
-
- HSPA5
-
- heat shock protein family A member 5
-
- HSP
-
- heat shock protein
-
- HSPB1
-
- heat shock protein beta-1
-
- IFN-γ
-
- Interferon-gamma
-
- iNOS
-
- inducible nitric oxide synthase
-
- IR
-
- ionizing radiation
-
- KEAP1
-
- kelch-like ECH-associated protein 1
-
- LKB1
-
- liver kinase B1
-
- LOX
-
- lipoxygenase
-
- LPCAT3
-
- lysophosphatidylcholine acyltransferase 3
-
- MAPK
-
- mitogen-activated protein kinase
-
- MCT1
-
- monocarboxylate transporter 1
-
- Mdm2
-
- murine double minute 2
-
- MEK
-
- mitogen-activated protein kinase
-
- MM
-
- malignant mesothelioma
-
- MT
-
- metallothionein
-
- mtDNA
-
- mitochondrial DNA
-
- MTF1
-
- metal-regulated transcription factor 1
-
- mTOR
-
- mammalian target of rapamycin
-
- mTORC1
-
- mechanistic target of rapamycin complex 1
-
- MVB
-
- multivesicular body
-
- NADH
-
- reduced nicotinamide adenine dinucleotide
-
- NAD+
-
- nicotinamide adenine dinucleotide
-
- NADP+
-
- nicotinamide adenine dinucleotide phosphate
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate
-
- NCOA4
-
- nuclear receptor coactivator 4
-
- NFS1
-
- nitrogen fixation 1
-
- Nox4
-
- NADPH oxidase 4
-
- NOXA
-
- NADPH oxidase activator 1
-
- NRF2
-
- nuclear factor erythroid 2-related factor 2
-
- NSCLC
-
- non-small cell lung cancer
-
- oxPC
-
- oxidized phosphatidylcholine
-
- oxPE
-
- oxidized phosphatidylethanolamine
-
- PCBP1
-
- poly (rC) binding protein 1
-
- PDAC
-
- pancreatic ductal carcinoma cell
-
- PDGFR
-
- platelet-derived growth factor receptor
-
- PDK4
-
- pyruvate dehydrogenase kinase 4
-
- PDT
-
- photodynamic therapy
-
- PE
-
- phosphatidylethanolamine
-
- PERK
-
- PKR-like endoplasmic reticulum kinase
-
- PI3K
-
- phosphatidylinositol-3-kinase
-
- PKC
-
- protein kinase C
-
- PPP
-
- pentose phosphate pathway
-
- PSTK
-
- phosphoseryl-tRNA kinase
-
- PTEN
-
- phosphatase and tensin homolog
-
- PUFA
-
- polyunsaturated fatty acid
-
- PUFA-PL
-
- polyunsaturated fatty-acid-containing phospholipid
-
- PUFA-PL-OOH
-
- PUFA-PL peroxidation
-
- PUMA
-
- p53 upregulated modulator of apoptosis
-
- RNS
-
- reactive nitrogen species
-
- ROS
-
- reactive oxygen species
-
- RO•
-
- lipid alkoxy
-
- RSL3
-
- RAS-selective-lethal 3
-
- S1R
-
- sigma-1 receptor
-
- SAPE-OOH
-
- 1-stearoyl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine
-
- SAS
-
- sulfasalazine
-
- SAT1
-
- spermidine/spermine N1-acetyltransferase
-
- SCD1
-
- stearoyl-coenzyme A desaturase-1
-
- sHSP
-
- small heat shock protein
-
- SLC3A2
-
- solute carrier family 3 member 2
-
- SLC7A11
-
- cystine glutamate transport receptor
-
- sMAF
-
- small Maf proteins
-
- SREBP1
-
- sterol regulatory element-binding protein 1
-
- SSB
-
- single-strand break
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- STEAP3
-
- metalloreductase
-
- STING1/TMEM173
-
- stimulator of interferon response cGAMP interactor 1
-
- TCA
-
- tricarboxylic acid
-
- TFAM
-
- transcription factor A, mitochondrial
-
- TFH
-
- follicular helper T
-
- TFR1
-
- transferrin receptor 1
-
- TLR2
-
- toll-like receptor 2
-
- TMZ
-
- temozolomide
-
- TrxR
-
- thioredoxin reductase
-
- TSC2
-
- tuberous sclerosis complex 2
-
- TXNRD
-
- thioredoxin reductase
-
- UPR
-
- unfolded protein response
-
- USP7
-
- ubiquitin-specific protease 7
-
- UV
-
- ultraviolet
-
- VDAC
-
- voltage-dependent anion channel
-
- VEGFR
-
- vascular endothelial growth factor receptor
-
- YAP
-
- yes-associated protein
-
- α-KG
-
- α-ketoglutarate
1 INTRODUCTION
The balance between cell proliferation and cell death is critical for maintaining tissue homeostasis and preventing the onset of malignancies. The cellular stress response is a complex process that involves the activation of survival pathways and the initiation of cell death pathways that eventually eliminates damaged cells [1]. Damaged and unnecessary cells can be eliminated by activation of apoptosis and other forms of programmed cell death, such as autophagic cell death, necroptosis, pyroptosis, and ferroptosis [2]. Maladaptation to stressful situations is closely related to cancer initiation, progression, and therapeutic resistance. Autophagy and apoptosis are considered as stress response mechanisms involved in the determination of cell survival or death under stress conditions [3,4]. Recently, ferroptosis was reported to be associated with several kinds of stresses [5-9] and to participate in the regulation of antitumor therapeutic responses [10-12]. Ferroptosis is morphologically, biochemically, and genetically distinct from apoptosis, classic necrosis, and other forms of nonapoptotic cell death [13]. The requirements for iron and the accumulation of reactive oxygen species (ROS) are the defining features of ferroptosis. The complex interaction between lipid, iron, and cysteine metabolism is an essential regulator of this cell death pathway [13]. Inducers, inhibitors, genes, and pathways that regulate ferroptosis have been summarized in detail by Hassannia et al. [14]. This review focuses on the interconnection between stresses and ferroptosis, and discusses how this process contributes to tumor progression and affects antitumor therapies.
2 HALLMARKS OF FERROPTOSIS
In 2003, Dolma et al. [15] identified a novel compound with a selective lethal effect on tumor cells expressing RASV12 through synthetic lethal screening and named it erastin. In 2007, using affinity purification and mass spectrometry, mitochondrial voltage-dependent anion channels (VDACs) were identified as specific targets of erastin. Erastin binds with VDACs to activate a rapid, oxidative, nonapoptotic cell death process through the RAS-RAF-mitogen-activated protein kinase (MEK) signaling pathway [16]. Erastin also inhibits cystine glutamate transport receptor (SLC7A11)-mediated cystine uptake to trigger iron-dependent cell death [13]. In 2008, Yang and Stockwell [17] discovered another RAS-selective lethal small molecule RAS-selective-lethal 3 (RSL3), which activates a similar form of cell death unrelated to VDACs or SLC7A11. Iron-chelating agents can significantly inhibit RSL3-induced cell death. In 2012, Dixon et al. officially named this unique iron-dependent, nonapoptotic cell death as ferroptosis, which is morphologically, biochemically, and genetically distinct from apoptosis, necrosis, and autophagy [13]. Morphologically, ferroptosis has neither typical apoptotic features such as chromatin condensation and marginalization nor typical necrotic features such as organelle swelling and rupture of the plasma membrane and has no typical autophagy features such as the formation of double-membrane enclosed vesicles [13]. Ferroptosis mainly manifests as reduced mitochondrial volume, increased membrane density, and reduced or absent mitochondrial cristae [13,16]. Biochemically, glutathione (GSH) depletion, glutathione peroxidase 4 (GPX4) activity inhibition and subsequent accumulation of lipid ROS specifically trigger ferroptosis [13]. Ferroptosis is a biological process regulated by multiple genes and pathways and is mainly related to iron metabolism, energy metabolism, lipid synthesis, and oxidative stress [13]. Iron accumulation and oxidation of polyunsaturated fatty acid-containing phospholipids can be considered as the most crucial ferroptosis features.
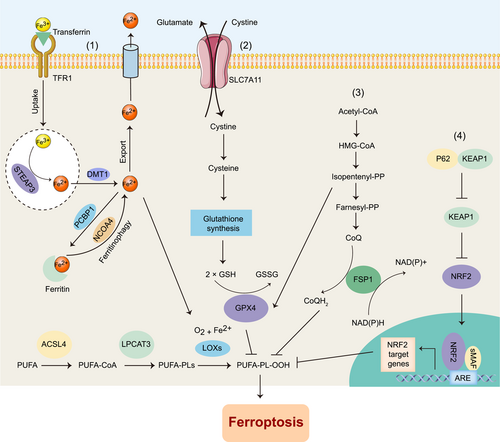
2.1 Iron accumulation
Ferroptosis is an iron-dependent form of nonapoptotic cell death. Free iron is a substance necessary for the accumulation of lipid peroxides and the execution of ferroptosis. Fe3+ is transported into cells through transferrin receptor 1 (TFR1)-mediated transferrin-iron complexes and then reduced to Fe2+ by STEAP3 metalloreductase (STEAP3) in endosomes. Downregulation of the import of transferrin-iron complexes suppresses ferroptosis by limiting the uptake of iron [18]. As an iron chaperone, poly (rC) binding protein 1 (PCBP1) delivers iron to ferritin, the iron storage protein [19]. In PCBP1-deleted mouse hepatocytes, unchaperoned iron leads to ROS production, resulting in lipid peroxidation and triggering the ferroptotic cell-death pathway [20]. Ferritin can be degraded through ferritinophagy (similar to autophagy) mediated by nuclear receptor coactivator 4 (NCOA4), which maintains intracellular iron homeostasis by facilitating ferritin iron storage or release according to demand [21-25]. Genetic inhibition of NCOA4 inhibited ferritin degradation and suppressed ferroptosis, presumably due to reduced intracellular free iron pools [25]. Nitrogen fixation 1 (NFS1) derives sulfur from cysteine to biosynthesize iron–sulfur clusters, which are required cofactors for several human enzymes. NFS1 depletion sensitized cells to ferroptosis by increasing the accumulation of free, redox-active iron [26]. Lipoxygenases (LOXs) are nonheme iron-containing enzymes [27]. Iron in the active site of a LOX plays an essential role in catalyzing the formation of lipid hydroperoxides from polyunsaturated fatty acids (PUFAs), thereby producing toxic lipid hydroperoxides. Iron chelators such as deferoxamine and deferiprone are currently used to treat iron overload diseases by preventing ferroptosis by removing catalytic iron from LOXs [28]. Therefore, proteins involved in iron uptake regulation, export, and storage may affect the induction of ferroptosis by regulating redox-active iron accumulation.
2.2 Oxidation of polyunsaturated fatty acid-containing phospholipids
Lipid metabolism is closely related to ferroptosis. Both erastin- and RSL3-induced cell death involve the iron-dependent accumulation of lipid ROS [29]. During ferroptosis, PUFAs are the lipids most susceptible to peroxidation [30]. Esterification of PUFAs into phospholipids requires acyl-CoA synthetase to form PUFA-CoA [31]. Phosphatidylethanolamine (PE) containing arachidonic acid (AA) or its derivative adrenaline (AdA) is a critical phospholipid that causes ferroptosis in cells. Genetic or pharmacological targeting of acyl-CoA synthetase long-chain family member 4 (ACSL4) or lysophosphatidylcholine acyltransferase 3 (LPCAT3) inhibited the esterification of AA or AdA to PE and reduced the accumulation of lipid peroxide substrates in the cells, thereby inhibiting ferroptosis. Under the catalysis of LOXs, polyunsaturated fatty-acid-containing phospholipids (PUFA-PLs) can further play an oxidative role and ultimately induce ferroptosis [30,32]. Glutaminolysis is a glutamine-fueled intracellular metabolic adaptation pathway that catabolizes glutamine to generate adenosine triphosphate (ATP) and lactate, yielding glutamate and ammonia. Glutamate is then converted into α-ketoglutarate (α-KG), which interacts with the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, and citrate-associated fatty acid metabolism to augment ROS and lipid synthesis [18]. Besides, glutamine derived aspartate can assist the production of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) and decrease ROS level [33].
General ferroptosis regulatory countermeasures can be divided into four categories [34] (Figure 1). (1) One category is inhibition of the Fenton reaction, a process in which peroxides and Fe2+ react to generate soluble hydroxyl (HO•) or lipid alkoxy (RO•) radicals; (2) Another category is the promotion of GSH production and free radical scavenging. Cysteine is a crucial substrate for GSH synthesis to support the cellular antioxidant system. In addition to obtaining cysteine through SLC7A11-mediated uptake, cellular cysteine can also be synthesized from methionine through the transsulfuration pathway [35]. CARS encodes Cysteinyl-tRNA synthetase, an enzyme that is involved in the charging of tRNAs with cysteine for protein translation [36]. Loss of CARS suppresses the ferroptosis induced by erastin through the activation of the transsulfuration pathway [31]. When facing extracellular cysteine limitation, cysteine biosynthesis mediated by the transsulfuration pathway can provide enough cysteine to maintain tumor cell proliferation [35]. (3) Another category is repairing the damage of toxic lipid hydroperoxides. GPX4 is a key protein at the center of this repair network that reduces esterified oxidized fatty acids and cholesterol hydroperoxides. Ferroptosis suppressor protein 1 (FSP1) was found to be a ferroptosis inhibitor independent of the classic GPX4 signaling pathway. Myristoylation recruits FSP1 to the plasma membrane and acts as an oxidoreductase on the plasma membrane, reducing coenzyme Q10 (CoQ), inhibiting lipid peroxidation, and then inhibiting ferroptosis [37,38]. (4) The final category is the induction of adaptive responses to combat ferroptosis. Nuclear factor erythroid 2-related factor 2 (NRF2) plays a critical role in the induction of antioxidant response and has a central role in protecting cells from ferroptosis in cancers such as hepatocellular carcinoma (HCC), head and neck cancer, and gliomas [39,40]. Ferroptosis-inducing compounds, such as erastin or sorafenib, induce the expression and nuclear accumulation of NRF2 by increasing the interaction between Kelch-like ECH-associated protein 1 (KEAP1) and P62 [40].
3 CROSSTALK BETWEEN FERROPTOSIS AND OTHER FORMS OF CELL DEATH, p53 AS AN EXAMPLE
Although ferroptosis is morphologically, biochemically, and genetically distinct from other forms of cell death, studies on the relationship among them have never stopped. Various forms of cell death are closely interconnected and form a network to regulate cell survival under a diverse range of pathophysiological processes and stresses. Multiple evidence suggested that chemotherapeutic agents or combined strategies suppress tumors by inducing ferroptosis and other forms of death simultaneously. The combination of siramesine (a lysosomal disruptor) and lapatinib (a dual tyrosine kinase inhibitor) has been shown to synergistically induce breast cancer cell death caused by ferroptosis and autophagy at different periods of disease [41]. Sorafenib, a multikinase inhibitor for advanced HCCs, could decrease extracellular signal-regulated kinase (ERK) phosphorylation and induce apoptosis [42], and also induce ferroptosis by inhibiting the activity of the SLC7A11 in HCC Lines [43]. These evidence suggested that common regulatory factors or pathways may be involved in different types of cell death.
Various key regulators of autophagy or apoptosis have been reported to induce or inhibit the process of ferroptosis, such as p53 [44-46]. As a key regulatory protein in programmed cell death, p53 regulates cell death in various ways. When faced with diverse stress stimuli, including oncogene activation, DNA damage, or nutrient deprivation, p53 is stabilized and activated by a series of posttranslational modifications that free it from murine double minute 2 (Mdm2)-induced ubiquitination and degradation [47,48]. The function of stabilized p53 is very complex and cell or tissue type-dependent. The cytosolic and nuclear-accumulated p53 are both considered to be involved in apoptosis induction. Low Mdm2 activity could induce p53 monoubiquitination, which promotes p53 mitochondrial membrane localization and interaction with Bcl-XL and Bcl-2. Such effects stimulate the oligomerization of the proapoptotic factors Bcl-2 homologous antagonist/killer (BAK) and Bcl-2-associated X (BAX), driving the formation of pores in the mitochondrial membrane and cell apoptosis [49]. Nuclear p53 induces p53 upregulated modulator of apoptosis (PUMA), BAX, NADPH oxidase activator 1 (NOXA), and other gene transcription to promote cell apoptosis. Generally speaking, key coactivators recruitment (e.g., CBP/p300 and Tip60) to the target promoters and full acetylation of p53 is required for the irreversible apoptotic response [50]. As mentioned above (“Oncogenic stress” part), p53 acts as a double-edged sword in ferroptosis. It is also the case of p53 in autophagy regulation. On the one hand, p53 induces autophagy by several pathways including transcriptionally upregulating its downstream target genes, such as AMPK, tuberous sclerosis complex 2 (TSC2), phosphatase, and tensin homolog (PTEN), and so on, thereby suppressing mTOR activity and initiates autophagy [51]. On the other hand, p53 in the cytoplasm inhibits AMPK, activates mTOR and leads to autophagic suppression. More studies are needed to further explore how “ferroptotic,” “apoptotic” and “autophagic” processes work alone or synergistically to be integrated into a complete regulatory network. Studies should be performed to elucidate how key proteins or cell death regulatory pathways, such as p53, act as a “switch” to determine cell fate in response to different stress conditions. A deep understanding of the precisely regulatory mechanisms may provide new avenues for cancer treatment, for example, inducing ferroptosis-dependent translational modification of p53 when tumor cells are resistant to apoptotic or autophagic cell death.
4 FERROPTOSIS IN STRESS RESPONSES
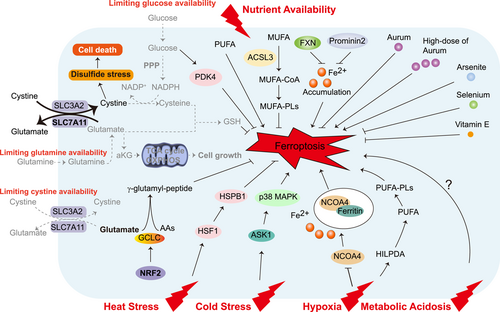
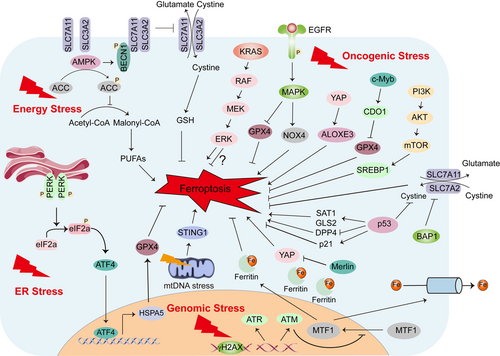
Cells can respond to stress in many ways, from activating survival pathways to triggering cell death to eliminating damaged cells. The initial response protects cells from stress-induced damage. However, if the harmful stimulus is not resolved, cell death signaling will be activated. Ferroptosis is an iron-dependent cell death driven by lipid peroxidation that is controlled by internal oxidative stress and antioxidant systems [52]. Ferroptosis itself is closely related to multiple extrinsic stresses enriched in the tumor microenvironment involved in nutrient availability, temperature, hypoxia, and metabolic acidosis (Figure 2) or cancer cell-intrinsic stresses such as energy stress, oncogenic stress, endoplasmic reticulum (ER) stress, genomic stress and so on (Figure 3). A deep understanding of the relationship between ferroptosis and various stresses will provide new ideas for the treatment of various refractory diseases, including cancers.
4.1 Extrinsic stress responses
4.1.1 Nutrient availability
Both normal and tumor cells require sufficient nutrients and energy to survive. However, the metabolic requirements of cancer cells and nutrient availability obtained by tumors from the environment may differ from those of normal cells. Nutrients available to tumors can directly affect not only tumor survival and proliferation, but also other genes and metabolic pathways [53]. Thus, by limiting the nutrient availability required by tumors in the environment and the intrinsic ability of cells to acquire and efficiently utilize these nutrients, it may be possible to modify tumor cell resistance to cell death, such as ferroptosis. Some intracellular nutrients (trace elements and vitamin E, etc.) closely related to the cellular antioxidant system, as well as exogenous nutrients such as glucose and amino acids, have been reported to be involved in the regulation of ferroptosis-related pathways [54].
Environmental stress from limiting or supplying exogenous nutrients such as glucose, amino acids, or fatty acids may sensitize tumor cells to ferroptosis. Glucose depletion inhibits the sensitivity of human pancreatic ductal carcinoma cell (PDAC) to ferroptosis. Glucose transporter SLC2A1-mediated glucose uptake promotes glycolysis, thereby promoting pyruvate oxidation and stimulating fatty acid synthesis, ultimately promoting lipid peroxidation-dependent ferroptosis. As a driver of ferroptosis resistance, SLC2A1-dependent pyruvate dehydrogenase kinase 4 (PDK4) promotes glucose-dependent ferroptosis resistance by inhibiting pyruvate dehydrogenase [5]. The cystine/glutamate antiporter SLC7A11 could transport cystine to promote the biosynthesis of GSH and maintain the redox balance, resulting in protection from ferroptotic cell death [55]. However, SLC7A11high cancer cells are highly dependent on glucose or glutamine for survival and are exquisitely sensitive to glucose or glutamine starvation-induced cell death [56-59]. In SLC7A11high cancer cells, a large amount of imported cystine is likely toxic due to its low solubility, and this imported cystine is quickly reduced to more soluble cysteine in the cytosol in a manner dependent on NADPH supplied from glucose via the pentose phosphate pathway [60]. Limiting glucose availability in SLC7A11high cancer cells results in NADPH depletion and disulfide stress-related cell death [59]. In addition, high glutamate export drives more glutamine uptake to activate glutaminase for glutamate replenishment, making cells more sensitive to glutamine starvation [60]. These studies indicated the complexity of nutrient availability on cell fate determination and suggested that the relationship between nutrient limitation and ferroptosis needs to be further explored. In addition to glucose and glutamine, Recent evidence suggests that other nutrients are closely related to ferroptosis. Non-small cell lung cancer (NSCLC) cells with high NRF2 expression protect themselves from cystine starvation-induced ferroptosis by enhancing γ-glutamyl-peptide synthesis to limit the accumulation of glutamate via a noncanonical activity of glutamate-cysteine ligase catalytic subunit (GCLC) [61]. Exogenous fatty acid levels affect ferroptosis susceptibility in a “double-edged” manner. Enrichment of PUFAs in the plasma membrane predisposes tumor cells to ferroptosis. Dietary intake of the PUFA dihomo-gamma-linolenic acid (DGLA; 20:3n−6) induces ferroptosis in Caenorhabditis elegans and human cells [62]. Moreover, dietary supplementation with n-3 and n-6 PUFAs selectively induces ferroptosis in tumor cells in an acidic environment [6]. In contrast to PUFAs, however, administration of MUFAs make cells entering a ferroptosis-resistant cell state [63]. Exogenous MUFAs are converted to MUFA-PLs in an acyl-CoA synthetase long-chain family member 3 (ACSL3)-dependent manner, then reducing PUFA incorporation into phospholipids, replacing PUFAs in membrane lipids, hindering lipid ROS accumulation and inhibiting ferroptosis. Furthermore, exogenous MUFAs also protect cells from apoptotic lipotoxicity caused by saturated fatty acid accumulation in an ACSL3-independent manner.
Some intracellular nutrients are also involved in the regulation of ferroptosis. Regulation of cellular iron levels by altering iron storage and export is critical for ferroptosis. Inhibition of mitochondrial protein frataxin (FXN) expression significantly enhances erastin-induced ferroptosis by accelerating the accumulation of free iron and lipid peroxidation [64]. In terms of regulating iron transport, prominin2 could inhibit ferroptosis by promoting the formation of ferritin-containing multivesicular bodies (MVBs) and exosomes to transport iron out of cells [65]. In addition, iron ions may coregulate iron homeostasis metabolism with other metal ions to cope with the stress brought by iron loading. The FDA-approved anti-rheumatoid arthritis drug auranofin (AUR) is a small molecule compound containing aurum that upregulates hepcidin by activating the NF-κB/IL-6/JAK-STAT signaling pathway to reduce iron overload. High-dose AUR inhibits the thioredoxin reductase (TXNRD) family of enzymes and leads to lipid peroxidation and ferroptosis [66]. Arsenite can trigger major ultrastructural damage in mitochondria and induce oxidative stress, which leads to testicular cell death by inducing ferroptosis [67]. Abundance of the trace element selenium also affects ferroptosis sensitivity [68]. GPX4 requires selenium for its biosynthesis, and dietary selenium supplementation promotes ferroptosis resistance by upregulating the abundance and activity of GPX4 [69]. Selenium-GPX4 could protect follicular helper T (TFH) cells from ferroptosis. In GPX4-deficient immunized mice, the number of TFH cells was significantly reduced and unable to produce high-affinity protective antibodies. Enhancing the expression of GPX4 in T cells by dietary selenium supplementation can enhance the number of TFH cells and improve the antibody immune response [70]. Dietary vitamin E supplementation is also effective in preventing ferroptosis. GPX4 and vitamin E synergistically maintain lipid redox balance and prevent ferroptosis in hematopoietic stem and progenitor cells [71].
4.1.2 Heat stress and cold stress
Cells are continuously exposed to various environmental signal fluctuations, including fluctuations in temperature and pH value, and so on. Maintaining a steady temperature is vital for life. A sudden temperature rise destroys important cell structures and interferes with basic cell functions [72]. In response to heat stress, cells activate heat shock proteins (HSPs). HSPs have a complex protective mechanism that prevents nonspecific protein aggregation and helps the protein maintain its inherent structure. Heat shock factors (HSFs) are transcription factors that regulate the synthesis of HSPs. Heat shock protein beta-1 (HSPB1) is a small heat shock protein (sHSP), also called mouse Hsp25 or human HSP27. Studies have found that activation of the heat shock factor 1 (HSF1)-HSPB1 pathway can negatively regulate erastin-induced ferroptosis [73]. Protein kinase C (PKC)-mediated HSPB1 phosphorylation negatively regulates ferroptosis by inhibiting iron uptake by cells and the production of lipid ROS. Gene knockdown or drug inhibition of the HSF1-HSPB1 pathway significantly improves the anticancer activity of erastin in vivo and in vitro, providing a basis for the combinational therapy targeting HSPB1 and ferroptosis for tumor treatment [73]. HSF1 also protects cardiomyocytes from palmitic acid-induced ferroptosis by maintaining cellular iron homeostasis and restoring GPX4 expression [74]. In addition, heat shock in plant cells causes ROS to accumulate and consumes GSH, which in turn triggers iron-dependent oxidative cell death similar to the ferroptosis in animal cells [75].
The body temperature of a warm-blooded animal is kept approximately at 37°C even in a cold environment. However, it is difficult for most cells to generate heat to maintain the intracellular temperature. Dramatic changes in the extracellular environment can usually cause severe damage to cells, leading to cell death. Among them, the precise molecular regulation mechanism of cold stress on the novel death mode-ferroptosis is still unclear. Cold stress-induced ferroptosis can be observed in multiple tumor cell lines such as A549, HepG2, and HT-1080. Apoptosis signal-regulating kinase 1 (ASK1) gene-deficient mice are sensitive to cold stress [76], and severe cold stress can activate the p38 mitogen-activated protein kinase (MAPK) pathway [77]. These findings suggest that ASK1 and MAPK signals may be involved in the cold stress response. Later, in 2017 a team from Japan found that sustained severe cold stress induces ferroptosis through the accumulation of lipid peroxides [78]. To cope with continuous exposure to an extremely low-temperature environment, lipid peroxides accumulate in a time-dependent manner and activate downstream ASK1-p38 MAPK pathways, thereby inducing ferroptosis. Ferroptosis inhibitors such as Fer-1, iron chelator deferoxamine (DFX), and p38 inhibitors are considered potential therapeutic drugs to prevent cold-induced damage. The link between cold stress and tumor growth was discovered more than 40 years ago [79], and given the discovery of the association between cold stress and ferroptosis, we need to focus on the regulation of cold stress-induced ferroptosis and thus on tumor growth in the future.
4.1.3 Hypoxia and metabolic acidosis
Hypoxia is one of the basic characteristics of solid malignant tumors and is closely related to tumor angiogenesis, metastasis, and drug resistance [80]. Tumor cells adapt to the anaerobic microenvironment through the release of tumor hypoxia-inducible factors (HIFs) [80]. The effect of hypoxia on ferroptosis is context-dependent. On the one hand, hypoxia reduces intracellular free iron by increasing ferritin expression to protect cells from ferroptosis [81]. As a hypoxia-responsive protein, carbonic anhydrase 9 (CA9) expression is significantly higher under hypoxia and correlates with increased catalytic Fe (II) in malignant mesothelioma (MM). CA9 inhibitor decreased (ferritin heavy chain/ferritin light chain) FTH/FTL and induced ferroptosis [82]. In this regard, reoxygenation may be an effective strategy to activate ferroptosis. Indeed, an Fe (VI)-based nano-oxygen release system activated by ultrasound can overcome the resistance of tumor cells to doxorubicin (DOX) induced by the hypoxic microenvironment by synergistically inducing apoptosis and ferroptosis in solid tumors [83]. The ferrate released by this system can effectively react with water and high levels of hydrogen peroxide and GSH in tumor cells to achieve tumor microenvironment-independent reoxygenation and GSH depletion. The metabolism of exogenous iron delivered via nanomedicine will initiate Fenton reactions, leading to the excessive production of ROS and iron-dependent cell death. GSH depletion inactivates GPX4, inhibits the reduction of lipid peroxides, thereby enhancing ferroptosis [83]. This evidence suggests that targeted induction of ferroptosis is a therapeutic approach for some hard-to-treat cancers. On the other hand, HIF signaling enhances lipid peroxidation to induce ferroptosis. Clear-cell carcinomas (CCCs) are highly aggressive malignancies distinguished by aberrant lipid and glycogen accumulation. CCCs are refractory to several anticancer therapies but show intrinsic vulnerability to ferroptosis in a hypoxia-dependent manner [8]. In renal CCCs, hypoxia-inducible factor 2 subunit α (HIF-2α) selectively enriches polyunsaturated lipids, the rate-limiting substrates for lipid peroxidation, by activating the expression of hypoxia-inducible, lipid droplet-associated protein (HILPDA). Additionally, autophagy-mediated degradation of aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL) (the core circadian clock protein) promotes the expression of egl nine homolog 2 (EGLN2) to destabilize hypoxia-inducible factor 1 subunit α (HIF-1α) and ultimately promote lipid peroxidation and ferroptosis [84].
Due to the poor oxygen perfusion and enhanced aerobic glycolysis present under hypoxic conditions, the overall tumor tissue is in an acidic microenvironment [85]. Tumor cells with higher glycolysis and acid resistance have more robust growth advantages. Accumulation of n-3 and n-6 PUFAs is toxic to acid-adapted cancer cells [6]. Ferroptosis is promoted when acidic cancer cells fail to buffer the enhanced uptake of PUFAs and expose themselves to the deleterious effects of lipid peroxidation. Most importantly, this dietary PUFAs supplementation has little effect on normal pH tissue cells. In addition, the ability of transferrin to bind iron is highly dependent on changes in pH value. At pH~7.4, iron effectively binds to transferrin outside cells, and when iron is delivered to acidic endosomes (pH~5.5) through receptor-mediated endocytosis, iron is dissociated from transferrin [28]. Currently, the exact molecular mechanism between ferroptosis and acidosis is unknown. Given the close relationship between iron and acidic environments, the effects of stress caused by acidosis on ferroptosis need to be further explored.
4.2 Intrinsic stress responses
4.2.1 Energy stress
Normal cells require sufficient energy to survive. Under pathological conditions, stress-induced metabolic changing triggers several adaptive responses to restore dynamic homeostasis and to maintain cellular function. One type of metabolic stress is energy stress, which is characterized by the consumption of intracellular ATP and a corresponding increase in intracellular adenosine monophosphate (AMP) levels. Energy stress induces an essential adaptive response mediated by AMP-activated protein kinase (AMPK), the cellular energy state sensor [86,87]. After AMPK is activated, ATP anabolism is inhibited to promote ATP catabolism, thereby restoring energy balance and maintaining cell survival. When facing long-term and excessive energy stress, ATP is excessively consumed, and this eventually induces apoptosis [86,87]. In a renal ischemia-reperfusion injury model, AMPK activation was found to inhibit ferroptosis, but this inhibition did not involve autophagy, the mechanistic target of rapamycin complex 1 (mTORC1) signaling, cystine uptake, or iron metabolism regulation [7]. Energy stress activates AMPK to phosphorylate and inactivate acetyl-CoA carboxylase (ACC), reducing the biosynthesis of PUFAs and other fatty acids, and ultimately inhibiting ferroptosis [7]. Liver kinase B1 (LKB1) is the main upstream kinase that activates AMPK in response to energy stress, and it is also one of the most common mutated tumor suppressors in NSCLC and cervical cancer [88]. Similarly, LKB1-AMPK axis phosphorylates ACC1 to negatively regulates fatty acid synthesis, protecting cells from lipid hydroperoxide accumulation and ferroptosis [89]. However, AMPK not only plays a tumor-promoting role by promoting the survival of tumor cells under metabolic or energy stress, but it can also exert a tumor-suppressing effect. AMPK mediates the phosphorylation of Beclin 1 (BECN1) to form a BECN1-SLC7A11 complex, directly block SLC7A11, and promotes ferroptosis through lipid peroxidation [90]. In subcutaneous and orthotopic tumor mouse models, genetically and pharmacologically activated BECN1 pathways increase ferroptosis. Hydroxycarboxylic acid receptor 1 (HCAR1) and monocarboxylate transporter 1 (MCT1) are both lactate transporters that mediate lactate absorption and promote ATP production to inactivate AMPK. In lactate-rich liver cancer cells, HCAR1/MCT1-mediated AMPK inactivation leads to the upregulation of sterol regulatory element-binding protein 1 (SREBP1) and downstream stearoyl-coenzyme A (CoA) desaturase-1 (SCD1), which enhance the production of anti-ferroptosis monounsaturated fatty acids [91]. These findings highlight that AMPK-mediated energy stress has complicated and cell type- or disease-dependent regulatory effects on ferroptosis.
4.2.2 ER stress
Under hypoxia, glucose deficiency, calcium imbalance, oxidative stress, injury, and other stress conditions, protein processing is affected, unfolded or misfolded proteins accumulate in the ER, and the unfolded protein response (UPR) occurs, causing ER stress [92,92]. The ER is the source of lipids for most membranes of other organelles and contains more than half of all the lipid bilayers in any given cell. Causes of ER stress include redox imbalance and lipid peroxidation. Upregulation of ER stress markers such as ChaC glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1), activating transcription factor 4 (ATF4), and phosphorylation of eukaryotic initiation factor 2α (eIF2α) can be observed during ferroptosis [94]. Indeed, Erastin and sorafenib, two reported ferroptosis agonists, induce ER stress by upregulating the ER stress response gene CHAC1 [55]. The heat shock protein family A (Hsp70) member 5 (HSPA5, also known as GRP78) is a member of the heat shock family, and it is mainly located in the ER or mitochondria. ATF4 upregulates HSPA5 in PDAC cells, which directly inhibits the degradation of GPX4 and inhibits the lipid peroxidation process of ferroptosis [95]. Genetic inhibition of the HSPA5-GPX4 pathway enhances the anticancer activity of gemcitabine by inducing ferroptosis [96]. The above evidence suggest that ER stress activation plays a negative effect on ferroptosis induction. Therefore, the combined use of ferroptosis inducers (FINs) and ER stress inhibitors may have great therapeutic significance for cancer treatments.
4.2.3 Oncogenic stress
Tumorigenesis is a multi-step and complex process with dysregulated oncogenic pathways involved. Recent evidence suggests that the oncogenic stress induced by hyperactivation of oncogenes or loss of suppressors in some cases is linked to ferroptosis that is associated with oncogenic transformation, tumor growth, and therapy response.
Mutant RAS is the first described oncogene that interplays with ferroptosis. Erastin and RSL3, the two classical FINs, were reported to have selective lethality against tumor cells expressing mutant RAS [15,17]. Inhibition KRAS or its downstream RAS-RAF-MEK-ERK pathway reverses the antitumor effect of erastin [16]. Moreover, KRAS-mutant lung adenocarcinoma cells are vulnerable to SLC7A11 inhibition [97]. These preclinical evidence supports the important interaction between KRAS mutation and ferroptosis, revealing a promising therapeutic strategy against KRAS-mutant tumors. Nevertheless, sensitivity profiling in 177 cancer cell lines revealed ferroptosis can be triggered by erastin regardless of the mutational status of RAS [29]. In contrast, ectopic expression of NRAS12V, KRAS12V, or HRAS12V protects RMS13 rhabdomyosarcoma cells from oxidative stress-induced ferroptotic death, suggesting the precise role of KRAS mutation in modulating ferroptosis is complex and requires further investigation. Notably, KRAS is not the only oncogenic signaling involved in regulating ferroptosis. It was reported that hyperactivation of several oncogenic signaling, including epidermal growth factor receptor (EGFR) pathway, Yes-associated protein (YAP) pathway, and c-Myb (a proto-oncogene protein) pathway, may elevate lipid ROS production and render cancer cells extremely susceptible to ferroptosis. EGFR-mutation sensitized NSCLC cells to ferroptosis in a MAPK signaling-dependent manner [98]. Oncogenic activation of YAP signaling sensitizes HCC cells to ferroptosis via arachidonate lipoxygenase 3 (ALOXE3) mediated lipid peroxidation accumulation [99]. Overexpression of c-Myb sensitizes gastric cancer (GC) cells to ferroptosis via upregulating cysteine dioxygenase type 1 (CDO1) expression and downregulating GPX4 expression [100]. However, hyperactive mutation of phosphatidylinositol-3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) signaling protects cancer cells from oxidative stress and suppresses ferroptosis via SREBP-mediated lipogenesis [9].
In addition to oncogenes, ferroptosis also crosstalk with bona fide tumor suppressors. Tumor suppressor p53 is spontaneously mutated or deleted in most human cancers. Despite the general important role of p53 in DNA repair, cell-cycle arrest, senescence, and apoptosis, it has been demonstrated that p53 act as a double-edged sword in ferroptosis. On the one hand, p53 enhances ferroptosis by inhibiting the expression of SLC7A11 or promoting the expression of spermidine/spermine N1-acetyltransferase (SAT1) and glutaminase 2 (GLS2). On the other hand, p53 inhibits ferroptosis by inhibiting dipeptidyl peptidase 4 (DPP4) activity or inducing cyclin-dependent kinase inhibitor 1A (CDKN1A)/p21 expression [101]. Notably, acetylation differentially regulates p53-mediated cell-cycle arrest, senescence, apoptosis, and ferroptosis. The acetylation-defective mutation p533KR (K117R + K161R + K162R) lost the ability to induce cell cycle arrest, senescence, and apoptosis, but retains the ability to promote ferroptosis and suppress tumor formation [44]. Loss of an additional acetylation site at K98 (namely p534KR) abolish its ability to induce ferroptosis, as well as its remaining tumor suppression activity [102], indicating ferroptosis contributes to p53-mediated tumor suppression. Like p53, tumor suppressor BRCA1-associated protein 1 (BAP1) is a tumor suppressor with frequent mutation or deletion in human cancers. Inactivation of BAP1 in cancer cells results in evaluated SLC7A11 expression, increased cystine uptake and GSH synthesis, and enhanced ferroptosis resistance, which contributes to tumor development. The mechanism how BAP1 suppresses SLC7A11 expression may partially attribute to the deubiquitinating H2A ubiquitination (H2Aub) on SLC7A11 [103]. Merlin, encoded by the neurofibromatosis type 2 gene, is a tumor suppressor frequently inactivated in MM. Genetic inactivation of Merlin renders MM cancer cells more sensitive to ferroptosis by promoting YAP activity, suggesting the involvement of Merlin-YAP signaling in dictating ferroptotic death [104].
Collectively, the above examples illustrate the context-dependent role of dysregulated oncogenic pathways in regulating ferroptosis. It is important to note that individual oncogene or tumor suppressors may play dual role in the control of ferroptotic death due to the tumor heterogeneity. More work is needed toward understanding the ferroptosis regulatory network so that optimal therapeutic approaches can be identified for future drug discovery efforts.
4.2.4 Genomic stress
The genome integrity is critical for the passage of genetic materials, and genomic DNA can be damaged by a variety of exogenous and endogenous sources such as replication errors, chemically induced adducts, ultraviolet (UV) induced damage, and single-strand (SSB) or double-strand (DSB) breaks caused by ionizing radiation (IR) or chemical reactions [105]. We refer to the genomic response induced by the above threats as genomic stress. In response to these threats, eukaryotes have evolved the DNA Damage Response (DDR) to sense DNA damage, repair DNA and process cells with damaged DNA to maintain genome integrity, and, when appropriate, execute cell death programs to remove unrepaired cells [106]. When cells are deficient in DDR and cannot repair damaged DNA, it can lead to genomic instability, one of the fundamental hallmarks of cancer [107]. Many DDR components including DNA damage sensor, signaling sensor, or downstream effector are involved in the regulation of ferroptosis [106]. Focusing on this interaction between DDR-related genomic stress and ferroptosis, a new type of cell death, is crucial in the study of cancer mechanisms.
Ataxia-telangiectasia mutated (ATM) and ATM- and Rad3-Related (ATR) are essential for DDR and maintenance of genome stability as kinases that sense DNA damage. Pharmacological or genetic inhibition of ATM protects a variety of cancer cells from ferroptosis induced by cystine deprivation or erastin. However, ATM inhibition rescues ferroptosis not through the canonical DNA damage pathway (ATM-P53/Chk2) but by increasing the expression of iron regulators involved in iron storage (ferritin heavy and light chains, FTH1 and FTL) and export (ferroportin, FPN1). Furthermore, ATM inhibition enhanced nuclear translocation of metal-regulated transcription factor 1 (MTF1), and genetic deletion of MTF-1 abolished ATM regulation of iron regulatory elements and resensitized cells to ferroptosis [108]. Zalcitabine induces the degradation of TFAM (transcription factor A, mitochondrial), which in turn leads to mitochondrial DNA stress and oxidative DNA damage. Mechanistically, Zalcitabine activates the stimulator of interferon response cGAMP interactor 1 (STING1/TMEM173)-mediated DNA sensing pathway leading to macroautophagy/autophagy-dependent ferroptotic cell death via lipid peroxidation [109].
In the DDR pathway, one of the key targets of ATM/ATR is p53 [106]. As an effector of DDR, p53 can respond to DNA damage, respond to oncogenic signals, and promote apoptosis or senescence. p53 can trigger protective pro-survival responses, such as temporary cell cycle arrest, DNA repair, or production of antioxidant proteins that maintain genome integrity and repair damaged cells [110]. The specific molecular regulation mechanism of DDR effector p53 on ferroptosis has been summarized in detail in the section “Oncogenic stress” and will not be repeated here.
5 FERROPTOSIS IN CANCER THERAPY RESPONSE
The pre-existing tumor heterogeneity and ongoing plasticity during therapy are the foundation of therapeutic resistance [111]. Tumor heterogeneity is connected with genomic alterations, epigenetic changes, and even tumor microenvironment stresses. Epithelial–mesenchymal transition (EMT) is a dynamic process and the main motor of tumor heterogeneity. Several stresses, such as hypoxia and metabolic stress have been found to act as upstream signal for EMT induction and accelerate tumor immune evasion, distal dissemination, and resistance to multiple treatments [112-115]. Counterintuitively, mesenchymal cancer cells, which are often resistant to diverse treatments, show susceptibility to ferroptosis [104]. In fact, cancer cells exhibit an increased iron demand to enable their quick growth, which makes them more vulnerable to ferroptosis [14]. From this perspective, deciphering the existing treatments in the regulation of ferroptosis and finding ways for ferroptosis induction could be new promising ways to kill therapy-resistant cancer cells. For instance, chemotherapeutic drugs may regulate ferroptosis in a context-dependent manner, and the combination of chemotherapy with ferroptosis induction could be a feasible way for cancer treatment. Notably, ferroptosis that happened in cancer cells and immune cells in tumor microenvironment may form a feedback network that determines the immunotherapy responses.
5.1 Inducing ferroptosis to sensitize chemotherapy and conquer chemoresistance
Chemotherapy is one of the most common treatment options for many cancers. Chemotherapy may inhibit ferroptosis or induce it, such effects could result in acquired resistance or therapeutic efficacy for cancer treatment. Cisplatin and paclitaxel promote the secretion of miR-522 from cancer-associated fibroblasts (CAFs) by activating the ubiquitin-specific protease 7 (USP7)/heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) axis. As a result, arachidonate lipoxygenase 15 (ALOX15) is inhibited, and ferroptosis in gastric cancer cells is reduced, eventually leading to acquired resistance to cisplatin [116]. Gemcitabine, one of the main chemotherapy drugs used for pancreatic cancer treatment, was reported to inhibit ferroptosis [96]. Gemcitabine increases the expression of HSPA5, which maintains GPX4 protein stability to inhibit ferroptosis. Genetic or pharmacological inhibition of HSPA5 enhanced gemcitabine sensitivity. Dihydroorotate dehydrogenase (DHODH) was reported to inhibit ferroptosis via inhibition of GPX4; combined treatment of brequinar (an inhibitor of DHODH) and sulfasalazine (SAS), a U.S. FDA-approved drug known to inhibit SLC7A11, induces ferroptosis and suppresses GPX4high tumor growth [117]. DMOCPTL, a derivative of natural product parthenolide, was reported to inhibit triple-negative breast cancer cell growth through inducing ubiquitination and degradation of GPX4, subsequently activating ferroptosis [118]. Dimethyl fumarate (DMF), a fumaric acid derivative, has been clinically used for the treatment for multiple sclerosis and psoriasis. Recently, DMF was found to mediate the induction of ferroptosis, providing a promising novel therapeutic option in the treatment of diffuse large B-cell lymphoma [119]. ALZ003, a curcumin analog, prompts F-box/LRR-repeat protein 2 (FBXL2)-mediated androgen receptor (AR) ubiquitination and subsequent degradation, thereby inducing ferroptosis by blocking AR-mediated GPX4 expression to increase lipid peroxidation [120]. ALZ003 was recently approved by the FDA as an orphan drug for the treatment of glioblastoma and may have the potential to become a drug for the treatment of recurrent glioblastoma. Several studies have explored the combination of chemotherapy with ferroptosis induction in the treatment of cancer. Temozolomide (TMZ) is an oral alkylating agent used to treat glioblastoma multiforme (GBM). Erastin makes GBM cells more sensitive to TMZ by inhibiting SLC7A11 and cystathionine gamma-lyase (CTH) activity, suggesting that the combination of TMZ and erastin may be a potential treatment strategy for GBM [121]. In addition to erastin, the combination of RSL3 with chemotherapy reagents has also been investigated. RSL3 together with a low concentration of paclitaxel synergistically induces ferroptosis in hypopharyngeal squamous carcinoma (HPSCC) cells harboring mutant p53 by upregulating mutant p53 expression, which mediates transcriptional regulation of SLC7A11 [122]. Buthionine sulfoximine (BSO) is an irreversible inhibitor of γ-glutamyl cysteine synthetase, the rate-limiting enzyme for GSH synthesis. Melphalan has been an integral part of multiple myeloma treatment. Increased GSH in multiple myeloma may mediate resistance to melphalan. Combining BSO with melphalan achieved complete responses (CRs) in three multiple myeloma xenograft models and significantly increased the median event-free survival compared with melphalan alone [123].
Chemoresistance often leads to tumor relapse, metastasis, and high rates of cancer-related mortality [124]. Inducing ferroptosis has been demonstrated to reverse drug resistance in chemoresistance cells. Many chemotherapeutic drugs kill tumor cells by inducing the excessive accumulation of ROS. However, some tumor cells have evolved mechanisms to escape ROS-mediated stress and gain tolerance to chemotherapeutic drugs [125]. Ferroptosis is mainly regulated by iron metabolism and lipid peroxidation signals. Interventions on these two processes may have therapeutic potential on therapy-resistant tumor cells. Cancer cells are more sensitive to iron deficiency and show higher utilization of iron than normal cells, thereby developing mechanisms to increase iron accumulation. High iron accumulation feature makes tumor cells more sensitive to ferroptosis, suggesting that induction of ferroptosis may provide new therapeutic opportunities against chemotherapy-resistant cancer cells. Inhibition of key ferroptosis-related lipid peroxidation factors, such as SLC7A11 and GPX4, may be also helpful for the eradication of cancer cells resistant to conventional chemotherapy [126]. SAS re-sensitized the cisplatin-resistant bladder cancer cells to cisplatin [10]. In addition, using CRISPR/Cas9 knockout screens, Chen et al. [127] identified phosphoseryl-tRNA kinase (PSTK), an essential RNA-dependent kinase, as a critical mediator of ferroptosis resistance in HCC cells. PSTK knockout could induce ferroptosis by suppressing GPX4 activity, GSH metabolism, and folate biosynthesis. Targeting PSTK may represent a novel approach to overcoming chemotherapy resistance via inducing ferroptosis in HCC [127]. Induction of cellular ferroptosis could also significantly reverse the oxaliplatin resistance of colorectal cancer by disrupting the KIF20A/NUAK1/PP1β/GPX4 pathway [128]. Moreover, chronic use of cisplatin-induced drug-resistant osteosarcoma cells by inhibiting ferroptosis. Ferroptosis agonists and signal transducer and activator of transcription 3 (STAT3) inhibitor re-activated ferroptosis by impairing STAT3/NRF2/GPX4 signaling and consequently enhanced the sensitivity of osteosarcoma cells to cisplatin [129]. These evidence revealed the important therapeutic role of inducing ferroptosis for conquering chemotherapy resistance.
Under some situations, ferroptosis induction may cause chemotherapy-related toxicity. DOX is a cytotoxic anthracycline antibiotic used to treat various cancers. However, DOX-induced cardiomyopathy limits its use for malignancies. DOX downregulates GPX4 and induces excessive lipid peroxidation in the mitochondria, which is a major cause of DOX cardiotoxicity [130]. Inhibition of ferroptosis with ferrostatin-1 could attenuate DOX-induced cardiomyocyte death. One should keep in mind that there are controversial conclusions on ferroptosis regulatory effect of chemoth, erapeutic agents, such as cisplatin [131], suggesting that chemotherapy agents may modulate ferroptosis in a context-dependent manner. Ferroptosis inhibition is not always detrimental for chemotherapy efficacy. Tertiary butylhydroquinone attenuates ferroptosis to protect 5-fluorouracil-induced intestinal mucositis [132]. Vitamin D receptor activation is protective against cisplatin-induced acute kidney injury by inhibiting ferroptosis [133]. These facts suggest that deeply understanding the regulatory effect and mechanism of chemotherapy agents on ferroptosis is necessary for rational design of combinational therapy to sensitize chemotherapy or reduce its toxicity.
5.2 Interconnection between radiotherapy and ferroptosis
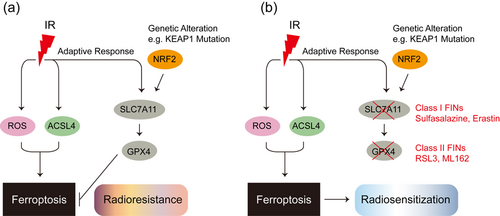
Radiation therapy is a cornerstone treatment for many cancers and is often combined with surgery or chemotherapy to delay tumor recurrence and improve overall survival. However, resistance remains a major factor leading to the failure of radiotherapy. Radiation therapy uses IR to break double-stranded DNA, which leads to cell cycle arrest, senescence, and various forms of cell death such as apoptosis, necrosis, autophagy, and mitotic catastrophe [134]. In addition to directly damaging DNA, IR also induces cell death through the radiolysis of cellular water and stimulation of oxidases and nitric oxide synthases to produce excess ROS and reactive nitrogen species (RNS) that destroy nucleic acids, proteins, and lipids, thereby indirectly causing cell damage [135]. High concentrations of antioxidants in cells are a huge obstacle to cancer radiotherapy efficacy. SAS inhibits the uptake of cystine by blocking SLC7A11, leading to GSH consumption, thereby making gliomas sensitive to gamma knife radiotherapy [136]; GSH consumption and inhibition of thioredoxin reductase (TrxR) activity enhance the radiation response in human breast cancer stem cells in vivo and in vitro through a thiol-dependent oxidative stress mechanism [137]. These evidence indicate that GSH depletion reduces radiation resistance in breast cancer and glioma cells, suggesting that FINs have potential as radiosensitizers. Indeed, in vitro, and in vivo data showed that FIN erastin could increase the sensitivity of tumor cells to X-ray irradiation via GSH starvation [11].
Both IR and ferroptosis are associated with excessive ROS, and the question of whether IR induces ferroptosis per se is inevitable. Studies have found that IR not only induces ROS production, but also increases the expression of the lipid metabolism enzyme ACSL4, which promotes lipid peroxidation and ferroptosis by regulating PUFA-PL biosynthesis [138]. In addition to inducing ferroptosis, IR also induces the expression of ferroptosis inhibitors, including SLC7A11 and GPX4, as an adaptive response, which promotes radiotherapy resistance by inhibiting ferroptosis. The inhibition of SLC7A11 or GPX4 by FINs can make radiation-resistant tumor cell lines and xenografts sensitive to IR [138]. Tumor suppressor KEAP1 targets NRF2 for ubiquitination and proteasomal degradation. Mutations in KEAP1-NRF2 signaling in lung or esophageal cancer lead to aberrant expression of SLC7A11, thereby inhibiting ferroptosis and promoting radioresistance [138]. Analysis of esophageal cancer patient samples before and after radiotherapy revealed that IR stress induces ferroptosis in cancer patients, which may help improve the prognosis of radiotherapy [138] (Figure 4).
5.3 Activating ferroptosis to overcome targeted therapy resistance
Targeted therapy drugs interfere with specific molecules (targets) that are involved in cancer cell growth and survival. Some targeted therapeutic agents also show ferroptosis regulating effects. Sorafenib, the targeted therapy for liver cancer, is currently considered to be the first-line medication for patients with advanced HCC. Sorafenib exerts antiangiogenic and antiproliferative effects by inhibiting the kinase activities of proteins such as Raf, vascular endothelial growth factor receptor (VEGFR), and the platelet-derived growth factor receptor (PDGFR)-β [139]. However, only approximately 30% of patients can benefit from sorafenib, and this population usually acquires drug resistance within 6 months. Metallothioneins (MTs) are a class of metal-binding proteins rich in cysteine in eukaryotic cells. MTs play an important role in detoxifying heavy metals and maintaining the redox homeostasis of cells. Sorafenib induces MT-1G transcription in an NRF2-dependent manner, resulting in therapeutic resistance [140]. Genetic and pharmacological inhibition of MT-1G enhances the anticancer activity of sorafenib in vitro and in tumor xenograft models. Sorafenib or erastin directly blocks SLC7A11 through AMPK-mediated formation of the BECN1-SLC7A11 complex and promotes ferroptosis [90]. Similarly, the sigma-1 receptor (S1R) can regulate ROS accumulation through NRF2, protecting HCC cells from sorafenib and subsequent ferroptosis. Genetic and pharmacological inhibition of S1R significantly improves the anticancer activity of sorafenib by downregulating the expression of GPX4, upregulating iron metabolism, and promoting ROS accumulation [141]. These findings provide new directions for overcoming sorafenib resistance in HCC patients.
Lapatinib is a dual tyrosine kinase inhibitor that interrupts the human epidermal growth factor receptor 2 (HER2)/neu and EGFR pathways. It is used as a first-line monotherapy for patients with HER2-positive metastatic breast cancer. However, the activation of compensatory pathways is known to contribute to lapatinib resistance [142]. Studies have shown that lapatinib together with siramesine increases FeCl3 levels, inducing ROS accumulation and ferroptosis in breast cancer cells [143]. Lapatinib treatment increased the expression of transferrin, which is responsible for the transportation of iron into cells. Moreover, siramesine treatment increased the expression of FPN1, which is responsible for the removal of iron from cells. These results provide new insights into how cancer cells are able to induce ferroptotic cell death and give hope that new therapeutic strategies can be developed to overcome apoptotic resistance in breast cancer.
The long-term efficacy of cetuximab, an EGFR-targeting antibody in patients with advanced rectal cancer, is often limited by the emergence of drug-resistant cells. The latest research found that vitamin C as a single agent or in combination with cetuximab can significantly downregulate the levels of reduced and total glutathione, causing increased ROS production and inducing ferroptotic cell death, thereby inhibiting the acquired resistance of rectal cancer cells to EGFR-targeted monoclonal antibody treatment [144]. Considering that high-dose vitamin C is known to be safe in cancer patients, using vitamin C to mobilize iron pools and induce ROS-mediated oxidative stress and ferroptosis in tumor cells may be a new strategy to fight EGFR-targeted therapy resistance. In addition, BRAF kinase inhibitors can cause drug-resistant melanoma cells to become more sensitive to ferroptosis induced by erastin, suggesting that ferroptosis inducers can be used as potential pre-combination therapy drugs to directly target dedifferentiation-based routes of acquired resistance [145].
5.4 Ferroptosis induction, a way to sensitize immunotherapy
Dying cells, especially apoptotic cells, communicate with immune cells by generating a series of “find me” or “eat me” signals, which allow immune cells to be recruited to the dying cell loci and promote the elimination of dead cells. In cancer, the engulfed dendritic cells or macrophages express tumor antigens by cross-presentation that activate tumor antigen-specific cytotoxic T lymphocytes. Friedmann and colleagues suggested that similar to apoptotic cells, cells that undergo ferroptosis may also release some “find me” signals, thereby attracting antigen-presenting cells (APCs) or other immune cells to the site of ferroptotic cells [146]. Lipidomic analyses of cells undergoing ferroptosis have revealed the accumulation of oxygenated lipid mediators, possibly generated through ALOX15. Although the role of free eicosanoids as signaling molecules modulating immune response has been reported, whether these lipid mediators acting as “find me” or “eat me” signals for ferroptotic cells has not been fully established. However, some indirect evidence are emerging. Lysophosphatidylcholine in the attraction of APCs has been previously demonstrated [147]. Another, ALOX15 limits the dendritic cells maturation via activation of NRF2 [148]. These evidence suggest that the lipid mediators released from the ferroptotic cells may also act as their “find me” or “eat me” signals. Direct evidence is that ferroptotic cells can be recognized and swallowed by macrophages, and the oxidized phospholipid, 1-stearoyl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), is a key “eat-me” signal on the ferroptotic cell surface [149]. Enriching the plasma membrane with SAPE-OOH increased the efficiency of phagocytosis of ferroptotic cells by macrophages through modulation of the macrophage surface receptor toll-like receptor 2 (TLR2). Except for lipid mediators, damage-associated molecular patterns (DAMPs) may also act as the “find me” or “eat me” signals. Ferroptotic cancer cells can release high mobility group box 1 (HMGB1) in an autophagy-dependent manner [150]. Once released outside of the cells, HMGB1 acquires immunostimulatory properties and contributes to the activation of the innate and adaptive immune systems by binding to pattern recognition receptors. Using RSL-3 tumor-specifically delivered nanoparticles to activate ferroptosis did promote effector T lymphocytes recruitment [151]. Collectively, ferroptotic cell death may be tightly connected with tumor immunogenicity and immunotherapy efficacy (Figure 5).
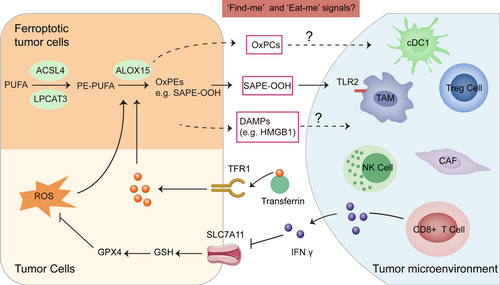
Immune cells can also step into ferroptotic cell death with different sensitivity. Such property may provide some opportunity for therapeutic manipulation. Macrophages are often divided into two broad categories: M1 and M2. The existence of immunosuppressive M2-like macrophages in the tumor microenvironment may be a crucial detrimental factor for antitumor immune response. M1-type polarized macrophages and microglia have high resistance to ferroptosis, while M2-type lacking inducible nitric oxide synthase (iNOS) show high sensitivity to ferroptosis [152]. Overall, iNOS may be an effective regulator of iron-dependent cell death upstream of GPX4. Thus, the regulation of ferroptosis by iNOS/NO may play an important role in controlling inflammation and the immune response. Photoimmunotherapy (e.g., photodynamic/photothermal therapy) is known to synergistically enhance the immune response to immunotherapy. However, excessively generated immunogenicity will cause serious inflammatory response syndromes. Researchers recently discovered that the biomimetic nanoparticle Fe3O4-SAS@PLT could sensitize cells to ferroptosis and generate mild immunogenicity, enhancing the response rate of noninflamed tumors to cancer immunotherapy [153]. Fe3O4-SAS@PLT induces ferroptosis in tumor cells by inhibiting the systemic cysteine/glutamate transporter. Nanoparticle-mediated ferroptosis can not only activate dendritic cell maturation, T cell activation, and infiltration, and induce tumor-specific immune responses; but also repolarize immunosuppressive M2-phenotype macrophages into the antitumor M1 Phenotype, thereby improving the in vivo therapeutic effect of PD-1 blockade therapy.
In turn, immune activation also contributes to ferroptosis induction. CD8+ T cells activated by tumor immunotherapy mainly induce tumor cell death through the perforin granzyme and Fas-Fas ligand pathways [154]. Interferon-gamma (IFN-γ) is the main effector molecule of activated CD8+ T cells. IFN-γ released from CD8+ T cells downregulates the expression of solute carrier family 3 member 2 (SLC3A2) and SLC7A11, two subunits of the glutamate-cystine antiporter system, impairs the uptake of cystine by tumor cells, and consequently, promotes tumor cell lipid peroxidation and ferroptosis [12]. Moreover, immunotherapy also sensitizes tumors to radiotherapy by promoting tumor cell ferroptosis [155]. Similarly, photodynamic therapy (PDT) promotes the recruitment of immune cells and the secretion of IFN-γ, which make tumor cells more sensitive to ferroptosis [156]. Therefore, T cell-promoted tumor ferroptosis is a novel antitumor mechanism of immunotherapy and targeting ferroptosis in combination with checkpoint blockade is a potential therapeutic approach (Figure 5). In summary, ferroptosis that happened in cancer cells and immune cells may form a feedback network that determines the immunotherapy responses.
6 FUTURE PERSPECTIVES
Ferroptosis is the result of the imbalance between cellular metabolic processes (e.g., lipid metabolism and iron handling). Alterations in the expression and activity of proteins or pathways controlling the levels, transport, storage, and metabolism of iron, PUFAs, cystine, cysteine, GSH, glutamine, and selenium will all influence the sensitivity of ferroptosis. Indeed, several ferroptosis inducers can cause tumor cell death in vivo. For clinical transformation, identifying approaches to determine which tumor types or states would most benefit from proferroptotic therapy is crucial. In addition, ferroptosis may play a dual role in cancer. In terms of tumor suppression, ferroptosis not only promotes tumor cell death, but also enhances tumor immunogenicity. In terms of tumor promotion, oxidized lipid mediators released from ferroptotic cancer cells may cause damage to dendritic cell maturation as well as natural killer cell or CD8+ T cell activation, thereby destroying antitumor immunity [148,157,158]. It is conceivable that if sufficient levels of mediators are achieved, the antitumor effect may shift to an immunosuppressive response. In this regard, how to control the potential adverse effects of ferroptosis in clinical cancer settings should be taken into account. More detailed molecular regulatory mechanisms and real-time diagnostic detection tools are needed to improve proferroptosis strategies and progress toward oncology clinical translation.
The clinical efficacy of traditional cancer therapies is far from satisfactory. Ferroptosis is attracting remarkable attention as a new therapeutic target for cancer treatment, especially with the rapid growth of the bionanotechnology field [159]. In the field of emerging therapies for cancer treatment, iron nanomaterials such as iron nanometallic glass and iron-based metal–organic frameworks wrapped with ferroptosis inducers have been widely used as anticancer drugs. Therefore, it is worth noting that the usage of biomaterials in cancer therapy opens up new ways and perspectives to overcome traditional cancer therapy resistance by targeting ferroptosis.
AUTHOR CONTRIBUTIONS
Fang Hua and Bing Cui conceived and participated in the overall design, supervision, and coordination of the manuscript. Cheng Zhang and Jiao-jiao Yu collated both written and verbal input from all authors and wrote the manuscript. All authors contributed ideas and assisted with editing for accuracy and clarity.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key R&D Program of China (2017YFA0205400), the National Natural Science Foundation of China (81973344 to FH; 81874316 to BC and 82173379 to JJY), CAMS Innovation Fund for Medical Sciences (2021-I2M-1-021 to FH and SS), Peking Union Medical College Graduate Innovation Fund (2019-1007-24 to CZ), Central Public-interest Scientific Institution Basal Research Fund (2018PT35004), and Beijing Outstanding Young Scientist Program (BJJWZYJH01201910023028). The authors sincerely apologize for not being able to keep as many important primary papers in the References because of strict space constraints.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest except Professor Fang Hua, who is a member of Cancer Innovation Editorial Board. To minimize bias, she was excluded from all editorial decision-making related to the acceptance of this article for publication.
ETHICS STATEMENT
None.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
This review did not generate new unique datasets.




