Research progress in the establishment of pancreatic cancer models and preclinical applications
Abstract
Pancreatic cancer (PC) is a highly malignant tumor in the digestive system. The transformation of tissue from normal to pancreatic intraepithelial neoplasm is driven by certain oncogenes, among which the mutation rate of the KRAS gene is as high as 90%. Currently, PC has limited treatment options, low therapeutic effects, and poor prognosis. Thus, more effective methods to combat PC are urgently needed. Some models that can more accurately reflect the biological behaviors and genomic characteristics of PC, such as its morphology, pathology, proliferation, and invasion, are being continuously developed. These include genetic engineering models, orthotopic xenograft models, and heterotopic xenograft models. Using these PC models, scientists have further verified promising drugs and potential therapeutic targets for PC treatment. This is of great significance for limiting the progression of PC with clinical intervention, improving patient outcomes, and improving survival rates.
Abbreviations
-
- APLP2
-
- Amyloid precursor-like protein 2
-
- BJO
-
- Brucea javanica
-
- CEA
-
- carcinoembryonic antigen
-
- EGF
-
- epidermal growth factor
-
- EMT
-
- epithelial-mesenchymal transition
-
- ERK
-
- extracellular signal–regulated kinase
-
- Exos
-
- exosomes
-
- GEMMs
-
- genetically engineered mouse models
-
- HDAC
-
- histone deacetylase
-
- IL
-
- interleukin
-
- IL2RG
-
- interleukin 2 receptor subunit gamma
-
- KPF
-
- Pdx1FlpOki; FSF-KRASG12D/+; p53frt/frt
-
- MDAs-ST/OT
-
- mouse-derived subcutaneous/orthotopic allograft tumor model
-
- MEKK
-
- mitogen-activated protein kinase kinase
-
- MET
-
- methionine
-
- MR
-
- methionine restriction
-
- MUC1
-
- Mucin 1
-
- NDAT
-
- nanodiamino-tetrac
-
- NLRP3
-
- nucleotide-binding domain leucine-rich repeat containing protein 3
-
- PAK4
-
- P21-activated kinase 4
-
- PanIN
-
- pancreatic intraepithelial neoplasm
-
- PC
-
- Pancreatic cancer
-
- PCAA
-
- pancreatic cancer-associated antigen
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PDOX
-
- patient-derived orthotopic xenograft
-
- PLB
-
- percutaneous liver gun biopsy
-
- PLGA
-
- poly(lactic-co-glycolic acid)
-
- PSC
-
- pancreatic stellate cell
-
- rMETase
-
- recombinant methioninase
-
- SAL
-
- salinmycin
-
- SHH
-
- sonic Hedgehog
-
- siPAK4
-
- PAK4-specific siRNA
-
- SLC6A14
-
- solute carrier family 6 member 14
-
- TGFβR
-
- transforming growth factor β receptor
-
- TRA
-
- trametinib
-
- TRAIL-R2
-
- TNF-related apoptosis-induced ligand receptor-2
-
- VEGF
-
- vascular endothelial growth factor
1 INTRODUCTION
Pancreatic cancer (PC) is a malignant tumor of the digestive system. Because this cancer has no obvious symptoms during its early stage, 80% of patients are already in the locally advanced stage or have distant metastasis at the time of diagnosis, preventing the possibility of radical surgical intervention. Although there has been consistent research on the etiology and treatment of PC over the past decade, the 5-year survival rate has not been significantly improved. For patients with locally advanced or metastatic PC, current radiotherapies and chemotherapies do not significantly improve prognosis [1]. According to statistics, the PC mortality rate ranks sixth among all malignant tumors in China [2]. Furthermore, population growth, aging, and lifestyle changes will likely continue to increase the incidence of PC over the next few years [3].
Among the histological and molecular features of pancreatic carcinogenesis, pancreatic intraepithelial neoplasm (PanIN) is a key factor in cancer formation. Driven by mutations in genes, such as KRAS, TP53, P16/CDKN2A, and SMAD4, PanIN progressively develops into PC, with most cases being categorized as pancreatic ductal adenocarcinoma (PDAC) with exocrine features of the pancreas [4, 5]. Among these mutated genes, the KRAS gene mutation rate is as high as 90%, making it the earliest and most common genetic event known to be involved in PC [6]. In addition, abnormal activation of certain signaling pathways [7], irregular protein expression levels [8, 9], and changes in the tumor microenvironment [10] all play roles in promoting the occurrence and development of PC. Therefore, to implement more comprehensive treatment options and obtain better prognoses for PC patients, further exploration and understanding of the molecular mechanisms of this disease are needed.
Currently, many preclinical models of PC have been established, such as transgenic, xenograft, and allograft models [11-14], all of which have important implications for studying PC. However, to more accurately reflect the biological behaviors of PC cells, such as morphology, pathology, proliferation, invasion, and genomic characteristics, new models are constantly being developed. This article reviews the new PC models established in recent years and how they can be used to validate drug candidates and novel therapeutic targets that may help treat PC.
2 ESTABLISHMENT OF A NEW PC MODEL
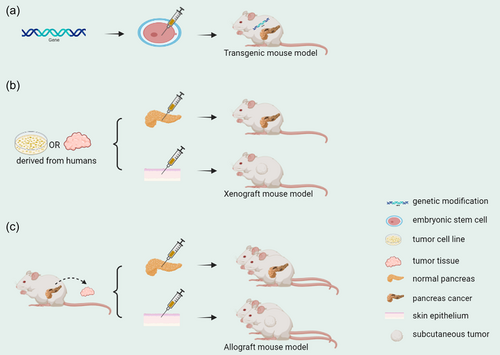
2.1 Transgenic mouse model
At present, genetically engineered mouse models (GEMMs) have great advantages for studying PC initiation, progression, metastasis, and therapy [14, 15]. These models use gene editing technology to knock out or overexpress specific genes in mouse embryonic stem cells, after which they develop normally in individual animals. After screening the offspring, certain mice can have the genetic modification at birth. Common GEMMs have been developed for KRAS activating mutations and deletions or mutations in other PC suppressors [16]. However, those models rely on epithelial-specific Cre-mediated recombination to induce tumor progression. Therefore, the unique Cre-loxP recombination present in mesenchymal cells does not apply here, which limits the study of mesenchymal genetics in tumorigenesis and progression in vivo. Wu et al. addressed this challenge by combining an alternative recombinase system in epithelial cells with Cre-loxP in stromal cells using the codon-optimized site-specific recombinase Flp (called FlpO) [17, 18], which has greatly increased reliability. They established a new Pdx1FlpO knock-in mouse line in the LSL-KRASG12D/+; LSL-p53R172H/+; PDX-1-Cre mouse model. The FlpO gene was specifically inserted into the transcriptional start site of the pancreas-duodenal homeobox 1 gene, making it a mouse model where pancreatic epithelial cells under normal conditions can also express FlpO recombinase. In the above model, the Frt locus can be specifically recombined in pancreatic epithelial cells. When that mouse line was mated with the Frt-STOP-Frt (FSF) KRASG12D and p53frt/frt mouse line, the Pdx1FlpOki; FSF-KRASG12D/+; p53frt/frt (KPF) mouse model was obtained. In the KPF mouse model, the KRAS mutation can simultaneously lead to the activation of Pdx1FlpO and loss of p53, resulting in a series of PDAC pathological changes, such as ductal carcinoma and PanIN. This can be combined with arbitrary stroma-specific Cre and, under certain conditions, its genes can also be targeted for modification. Therefore, this model will be a valuable tool to study stroma tissue-specific functions in the pancreatic tumor microenvironment [19].
2.2 Xenograft mouse model
Tumor xenograft mouse models have been widely used in preclinical research for more than a decade [20, 21]. These models are formed by transplanting cultured human tumor cells or human tumor tissue into immunodeficient mice. They have the advantage of mimicking genetic and epigenetic abnormalities present in tumors and are used to develop individualized molecular therapeutic strategies [22]. There are two types of tumor xenograft mouse models depending on where the graft is implanted in the mouse: the orthotopic and heterotopic models.
The orthotopic model is achieved by inoculating human tumor cell lines or patient-derived cancer tissues directly into the corresponding organs of mice, then inducing tumor growth in the organs in vivo. To better represent the PC phenotype and reflect the results of clinical trials, Higuchi et al. established a SUIT-2 orthotopic PC mouse model. The SUIT-2 cell line, which was derived from human PC metastatic liver tumors, was monolayered and fragmented in culture, with a doubling time of approximately 38.2 h. The modal number of chromosomes in SUIT-2 cells was 45, and the full count ranged from 34 to 176. The cells produced and released carcinoembryonic antigen (CEA) and CA19-9 [23]. In this study, SUIT-2 cell suspensions were injected orthotopically into the mouse pancreas. After observation, this model was found to resemble human PC in phenotypic progression and have the characteristics of extrapancreatic invasion, intraperitoneal spread, and metastasis to other hematogenous organs. After being orthotopically injected into the mouse pancreas, the SUIT-2 cells spread sequentially from the pancreas to the peritoneum, diaphragm, liver, and lungs, mimicking the natural process of human PC metastasis. Furthermore, the model accurately mimicked the effects of gemcitabine in human PC and detailed the effects of gemcitabine on primary and metastatic tumors. In the future, this model is expected to become the standard model for PC drug development [24]. In addition, studies indicate that cancer cell lines are gradually being replaced by patient-derived models [25]. Because the source of transplanted tumor cells is usually surgically resected samples, these samples are not fully representative of all cases because a large proportion of PC tumors cannot be surgically removed. Therefore, to establish a more representative model, Choi et al. performed percutaneous liver gun biopsy (PLB) in multiple patients with metastatic PDAC. The linear PLB samples were then implanted directly into the pancreas of athymic nude mice to establish a patient-derived orthotopic xenograft (PDOX) model. This type of model retains the characteristics of the primary tumor, both genetically and histologically. The first-generation cancer cells from PDOX-derived F1 tumor tissue were then grown into tumor organoids, and the epithelial cancer characteristics and KRAS mutations of the primary tumor were retained in these cells. The response of this PDOX model and organoids to gemcitabine correlated with the clinical outcome of the corresponding patient, showing that this PDOX-organoid model can successfully simulate the clinical details. Additionally, it has a more clinically representative drug response prediction ability and can enhance our understanding of the complicated nature of metastatic PDAC [26].
The heterotopic model also requires tumor cell lines or tumor tissues. However, in contrast to orthotopic transplantation models, these tumor cells and tissues are often engrafted into the subcutaneous tissue of mice rather than the normal anatomical site of the tumor's native organ. In the SUIT-2 cell line mentioned above, highly aggressive S2-013 cells with high CEA and CA19-9 levels can be isolated. These cells converge to form an epithelial monolayer and develop into a well-differentiated tubular adenocarcinoma [27]. However, the orthotopic transplantation mouse model established using S2-013 cells deviates from the histological structure of human PDAC [28]. For further study, Tanaka et al. established the S2-013 organoid model in vitro using the S2-013 cell line, human umbilical vein endothelial cells, and human bone marrow mesenchymal stem cells. S2-013 organoids were then subcutaneously injected into nude mice to form a heterotopic transplantation model. Compared with the S2-013 conventional heterotopic transplantation model, the S2-013-organoid heterotopic transplantation model showed no crater-like blood clot formation at the graft site, and the serum CA19-9 levels correlated with tumor volume. The grafts also exhibited cell morphology, histology, epithelial-mesenchymal transition (EMT), and the formation of a cancer stroma containing mature blood vessels similar to human PC. This model could be valuable for research that facilitates preclinical drug testing and biomarker discovery [29].
2.3 Allograft mouse model
In addition to promoting tumor progression, the PC tumor microenvironment can also increase drug resistance [30]. However, the actual progression of this could not be simulated because the mice in the transplanted tumor model are immunodeficient. At present, GEMMs are recognized as the most reliable in vivo models of PC, but their large-scale application is limited by their high expense, difficulty of establishment, and lack of terminal mouse numbers [31]. To ameliorate this limitation, Li et al. established a new in vivo PC model called the mouse-derived subcutaneous/orthotopic allograft tumor model (MDAs-ST/OT) using tumor tissue fragments from LSL-KRASG12D/+; Trp53fl/+; Pdx1-Cre transgenic mice for transplantation. MDAs accurately mimic the histopathology and various biological features of PDAC in immunocompetent organisms, including fibrosis, EMT, and the presence of invasion/metastasis-related markers. In addition, the immune cell recruitment process in PDAC was also simulated. MDAs are an effective model for studying PC progression and treatment, complementing the inadequacies of traditional genetic engineering models [32].
2.4 Other species models
Some scholars believe that current immunodeficiency mouse models do not truly replicate the characteristics of human PC, especially because they cannot mimic the multisite metastases observed during disease progression [33, 34]. Therefore, attention was given to other species to model PC. Among them, Syrian hamsters are highly similar in anatomy, physiology, and pathology to humans [35, 36]. Human interleukin (IL)-12 can reportedly effectively stimulate interferon-γ and tumor necrosis factor-α expression in hamster splenocytes and to exhibit toxicity in PC-bearing Syrian hamsters [37]. Therefore, Miao et al. created a novel immunodeficient Syrian hamster (designated ZZU001) with the IL-2 receptor subunit gamma (IL2RG) gene knocked out. The MIA-PaCa-2 cell line was subcutaneously or orthotopically transplanted into IL2RG knockout Syrian hamsters and severely immunodeficient mice. This cell line was derived from the pancreatic tissue of a patient with undifferentiated carcinoma. MIA-PaCa-2 cells have a 40-hour doubling time, and chromosomal counts revealed a highly aneuploid pattern. They contain measurable amounts of CEA, secrete a plasminogen activator, and are sensitive to L-asparaginase [38]. Compared with immunodeficient mice, ZZU001 hamsters developed metastases at multiple sites, and PC tissues showed a desmoplastic response in the stroma and EMT. This suggests that the IL2RG−/− Syrian hamster is a promising animal model to better understand the molecular mechanisms of tumorigenesis and to test novel therapies for aggressive tumors [39].
In addition, the zebrafish model has gradually become a strategy for implementing personalized medicine after the first experiment was reported in 2005 [40]. It has a short passage time, large number of offspring, transparency, and small size, making it more economical and practical than other in vivo cancer models. To illustrate the development of human PanIN, Park et al. used zebrafish to establish the first KRAS-initiated PC model. They used the CRE/Lox system to temporarily activate GAL4 transactivators in pancreatic progenitor cells and then used the GAL4/UAS system to amplify the transcription of target genes. This resulted in enhanced KRASG12V expression in the putative pancreatic ptf1a region, and the cells eventually developed into PanIN. The resulting PanIN was identified by immunohistochemical analysis as a characteristic pancreatic precancerous lesion and was strikingly similar to human PanIN. This provides an experimental and preclinical model system for studying the basic biology of PC and identifying potential therapeutic targets [41]. In addition, Franco et al. removed tumor tissue from PC surgical specimens, lysed and stained the tissue, transplanted it into zebrafish embryos, and incubated the embryos under suitable conditions. The model's response to chemotherapy regimens was then analyzed by monitoring the fluorescently stained areas of different chemotherapy regimens, demonstrating that this xenograft model is effective for evaluating such therapeutic approaches [42].
3 PRECLINICAL APPLICATION OF PC MODELS
To further understand the occurrence and development of PC and explore more effective treatment options, many scholars have used established PC models to conduct in-depth research. In addition, some drugs and therapeutic targets have been identified that have PC treatment potential.
3.1 Validation of potential therapeutic drugs for PC
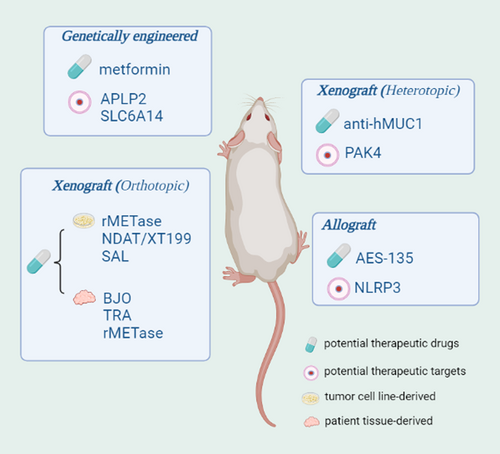
Metformin reportedly has some antitumor effects in a variety of cancers, including PC [43-45]. Qian et al. used the LSL-KRASG12D/+; Trp53fl/+; Pdx1-Cre transgenic mouse model of spontaneous PDAC to clarify the anticancer mechanism of metformin in PC. The study found that metformin exerts an antipancreatic stellate cell effect by reducing the expression levels of Sonic Hedgehog, which leads to downstream effects such as inhibited vascular endothelial growth factor production, reduced tumor angiogenesis, lower connective tissue proliferation rates, and increased sensitivity to the chemotherapy agent gemcitabine. Ultimately, metformin inhibits PDAC progression and prolongs survival in mice [46]. These results provide new evidence that metformin can be used as an adjuvant therapy strategy for PDAC.
Cancer cells are dependent on methionine (MET) because of the Hoffman effect [47, 48], while methionine restriction (MR) induces cell cycle arrest and apoptosis, and also inhibits tumor growth in vivo [49, 50]. According to reports, recombinant methioninase (rMETase) degrades methionine and has antitumor abilities both in vitro and in vivo [51]. Additionally, MR can promote TRAIL-R2 (TNF-related apoptosis-induced ligand receptor-2) expression, which is reportedly expressed at high levels in cancer cell lines and clinical tumor specimens [52]. Therefore, Yamamoto et al. further explored rMETase and TRAIL-R2 in the MIA-PaCa-2 [38] orthotopic PC mouse model. The results indicated that rMETase can enhance TRAIL-R2 expression levels in the tumor and promote the effects of the TRAIL agonist tigatuzumab, a drug that targets PC. This suggests that rMETase has clinical potential for treating PC [53]. In addition, integrins have been reported to affect tumor progression [54] and chemoresistance [55], for example, αvβ3 plays important roles in angiogenesis and the regulation of inflammation, and its receptor is highly expressed in cancer cells [56]. To clarify whether αvβ3 can act as a therapeutic target for PC, Coskun et al. investigated the function of the αvβ3 antagonists nanodiamino-tetrac (NDAT) and XT199 in a SUIT-2 [23] orthotopic PC mouse model. The αvβ3 antagonists significantly decreased the expression levels of the proinflammatory cytokines IL-1b, IL-6, and TNF-α, and increased the expression levels of the anti-inflammatory cytokine IL-10. In addition, NDAT or XT199 combined with cisplatin had more effective inhibitory effects on tumor growth and higher rates of tumor necrosis. These αvβ3 antagonists also improved tumor resistance to cisplatin and cisplatin-mediated motor dysfunction [57]. Other studies have shown that salinmycin (SAL) exhibits strong inhibitory activity in multidrug-resistant cancer cells [58] and various cancers [59-61]. To explore its role in PC, Daman et al. transported SAL into an ASPC-1 orthotopic PC mouse model with the aid of poly(lactic-co-glycolic acid) (PLGA) nanoparticles. ASPC-1 was derived from the ascites of a patient with PC that expressed PC-associated antigen (PCAA) and CEA. PCAA was different from CEA in its immunologic reactivity and distribution in its homologous tumoral tissues, and PCAA was predominantly associated with the proliferative phase of the malignant epithelium [62]. SAL upregulates the expression of β-catenin, E-cadherin, and transforming growth factor β receptor in tumors and inhibits tumor growth by selectively promoting tumor cell apoptosis. These results highlight SAL-loaded PLGA nanoparticles as a promising system for PC treatment [63]. In addition to the orthotopic model established using human PC cell lines, there are some new findings in PDOX models. Traditional Chinese medicine has been used as an important adjunct to cancer treatment [64]. The traditional Chinese medicine Brucea javanica can induce apoptosis of PC cells in vitro [65]. To further confirm its role in tumors in vivo, Yang et al. used B. javanica in a PDOX model to confirm that it can inhibit tumor growth in vivo and increase the survival rate of the model, suggesting that it has the potential to treat PC [66]. The mitogen-activated protein kinase kinase inhibitors trametinib and rMETase have also been demonstrated to significantly inhibit tumor progression in PDOX models [67] and improve tumor resistance to gemcitabine [68], respectively, after combined treatment with gemcitabine. Heterotopic transplantation models have also contributed to our knowledge of PC. Mucin 1 (MUC1) has been reported to be overexpressed in PC and may serve as a potential therapeutic target [69]. Wu et al. developed a monoclonal antibody (an anti-hMUC1 antibody) against the extracellular domain of the MUC1 subunit MUC1-C and evaluated its application in PC models. Capan-2 cells were obtained from pancreatic tissue from patients with ductal carcinoma of the pancreatic head. The cells showed characteristics of ductal epithelium, and the doubling time was 96 h. Chromosome studies showed a hypotetraploid pattern, and the cells exhibited a distinct enzyme phenotypic profile [70]. The anti-hMUC1 antibody could inhibit epidermal growth factor-mediated extracellular signal-regulated kinase phosphorylation and cyclin D1 expression, as well as tumor growth, in a Capan-2 heterotopic PC mouse model. This is expected to become an option for PC targeted therapy [71].
Previous research has also identified the presence of an oncogenic histone deacetylase (HDAC) in PC [72]. Although HDAC inhibitors can effectively kill PC cells in vitro, there are no related drugs approved for cancer treatment. Shouksmith et al. developed a novel HDAC inhibitor, AES-135, and determined that it could effectively inhibit tumor growth in an allograft model derived from KRASLSL.G12D/+; Trp53R172H/+; Elas-CreER mice and significantly extend the survival of the mice. Therefore, AES-135 is a promising drug candidate for further preclinical testing [73].
3.2 Validation of potential PC therapeutic targets
Amyloid precursor-like protein 2 (APLP2) has been reported to be increased in various human cancers [74-77]. Poelaert et al. found higher APLP2 expression levels in PC epithelial cells compared with PanIN and stromal cells, suggesting that the expression levels of APLP2 are associated with PC development. In the LSL KRASG12D/+; LSL-Trp53R172H/+; PDX-1-Cre transgenic mouse model, immunohistochemical analysis showed that APLP2 expression was significantly enhanced during the occurrence and development of PC, and knockout of APLP2 significantly prolonged survival and reduced metastatic progression. This suggests that APLP2 may be a potential therapeutic target in PC [78]. Similarly, the amino acid transporter solute carrier family 6 member 14 (SLC6A14) is also upregulated in different types of cancer, such as PC [79], colorectal cancer [80], breast cancer [81], and cervical cancer [82]. This finding was validated by Schniers et al. in the same genetically engineered mouse model described above. The results showed that deletion of SLC6A14 at the molecular level resulted in a decreased proliferative index and desmoplastic response without compensatory upregulation of any other amino transporters. In addition, tumor growth, tumor metastasis, and ascites accumulation were inhibited in the SLC6A14 knockout mice, which improved the overall survival rate of the mice [83]. This finding demonstrates that SLC6A14 is a viable drug target for PC, as well as any other cancer that overexpresses this transporter.
An elevated platelet-to-lymphocyte ratio in PC patients is a marker of decreased survival and poor prognosis [84]. The nucleotide-binding domain leucine-rich repeat containing protein 3 (NLRP3) inflammasome is an important inflammatory mechanism recently discovered in platelets that controls platelet activation and aggregation. Using the Panc02 orthotopic mouse model, Boone et al. found that knockdown of NLRP3 inhibited platelet activation, platelet aggregation, and tumor progression and significantly improved mouse survival. Panc02 cells were obtained from ductal adenocarcinoma tissue induced in the mouse pancreas and originated as a grade II tumor. After transplantation back into mice, the tumor retained a well-differentiated histological appearance (a grade III tumor appearance) and was a solid tumor, which allowed for effective evaluation of the metastasis rate [85]. Therefore, the platelet NLRP3 inflammasome plays an important role in the development of PC and may be a novel therapeutic target [86].
In addition, PC development is also associated with overexpression of the P21-activated kinase 4 (PAK4) gene [87]. To verify whether PAK4 can act as a therapeutic target for PC, Xu et al. transferred PAK4-specific siRNA (siPAK4) into PANC-1-cell-derived exosomes. The PANC-1-cell line was derived from human pancreatic ductal cell carcinoma and has a doubling time of 52 h. The G6PD activity was of the slow mobility or B type, and chromosome studies showed a modal number of 63 with three distinct marker chromosomes and a small ring chromosome [88]. Then, these PANC-1 cells were subcutaneously implanted into mice to establish a heterotopic transplantation model. Silencing of PAK4 resulted in delayed tumor growth in vivo and in vitro, and also improved mouse survival [89]. This study not only validates PAK4 as an effective RNA interference target for the treatment of PC, but also provides a rational design scheme for exosome-based cancer therapy (Figures 1 and 2, Tables 1–3).
| Type | Name | Advantage | Reference |
|---|---|---|---|
| GEMM | Pdx1FlpOki; FSF-KRASG12D/+;p53frt/frt (KPF) mouse model | Binds to substrate-specific Cre and can be used to conditionally modify target genes of interest | [19] |
| Xenograft mouse model | SUIT-2 mouse model | Similar to the phenotypic sequential progression of human pancreatic cancer | [24] |
| PLB-PDOX mouse model | More representative, preserving genetic alterations and histopathological features of the primary tumor | [26] | |
| S2-013 organoid mouse model | Cell morphology and histology are similar to human pancreatic cancer, with EMT and formation of a cancer stroma containing mature blood vessels | [29] | |
| Allograft mouse model | MDAs-ST/OT | Accurately mimicks the characteristics of PDAC and the recruitment of immune cells in immunocompetent organisms | [32] |
| Other species models | IL2RG-/- Syrian hamster | Multisite metastases occurred, and pancreatic cancer tissue showed desmoplastic responses in the stroma and EMT | [39] |
| Zebrafish model | More practical and economical than other in vivo cancer models | [41, 42] |
- Abbreviations: EMT, epithelial-mesenchymal transition; GEMM, genetically engineered mouse models; MDAs-ST/OT, mouse-derived subcutaneous/orthotopic allograft tumor models; PDOX, patient-derived orthotopic xenograft; PLB, percutaneous liver gun biopsy.
| Validation of potential therapeutic drugs | |||
|---|---|---|---|
| Model | Drug | Resulting effects on cancer | Reference |
| LSL-KRASG12D/+; Trp53fl/+; Pdx1-Cre mouse model | Metformin | Inhibit VEGF production, reduce tumor angiogenesis, reduce desmoplastic response, and enhance chemosensitivity to gemcitabine | [46] |
| MIA PaCa-2 orthotopic mouse model | rMETase | Enhance the expression level of TRAIL-R2 in the tumor, and promote the efficacy of the TRAIL agonist tigatuzumab targeting pancreatic cancer | [53] |
| SUIT-2 orthotopic mouse model | NDAT, XT199 | Increase anti-inflammatory cytokine expression, inhibit tumor growth, and improve tumor resistance to cisplatin | [57] |
| ASPC-1 orthotopic mouse model | SAL | Upregulates the expression of E-cadherin, β-catenin and TGFβR, and selectively promote tumor cell apoptosis | [63] |
| PDOX mouse model | BJO | Inhibit tumor growth in vivo and increase the survival rate of the model | [66] |
| TRA | Significantly inhibit tumor progression | [67] | |
| rMETase | Improve tumor resistance to gemcitabine | [68] | |
| Capan-2 heterotopic mouse model | Anti-hMUC1 antibody | Inhibit EGF-mediated ERK phosphorylation and cyclin D1 expression, and inhibit tumor growth | [71] |
| KRASLSL.G12D/+; Trp53R172H/+; Elas-CreER mouse-derived allograft models | AES-135 | Inhibit tumor growth and prolong survival | [73] |
| LSLKRSG12D/+; LSL-Trp53R172H/+; PDX-1-Cre mouse model | APLP2 | Significantly prolonged survival and reduced metastatic progression | [78] |
| SLC6A14 | Decreased the proliferative index and desmoplastic response | [83] | |
| Panc02 orthotopic mouse model | NLRP3 | Inhibited platelet activation, platelet aggregation, tumor progression, and significantly improved mouse survival | [86] |
| PANC-1 heterotopic mouse model | PAK4 | Delayed tumor growth and improved mouse survival | [89] |
- Abbreviations: AES-135, histone deacetylase inhibitor; APLP2, amyloid precursor-like protein 2; BJO, Brucea javanica; EGF, epidermal growth factor; ERK, extracellular signal–regulated kinase; NDAT and XT199, αvβ3 integrin antagonists; NLRP3, nucleotide-binding domain leucine-rich repeat containing protein 3; PAK4, P21-activated kinase 4; PDOX, patient-derived orthotopic xenograft; rMETase, recombinant methioninase; SAL, salinmycin; SLC6A14, solute carrier family 6 member 14; TGFβR, transforming growth factor β receptor; TRA, trametinib; TRAIL, TNF-related apoptosis-induced ligand; TRAIL-R2, TNF-related apoptosis-induced ligand receptor-2; VEGF, vascular endothelial growth factor.
| Name | Origin | Feature | Reference |
|---|---|---|---|
| SUIT-2 | Metastatic liver tumor of human pancreatic carcinoma | The cells grew in a monolayered sheet with a population doubling time of approximately 38.2 h. The cells had chromosome counts ranging from 34 to 176 with a modal number of 45 and produced and released CEA and CA19-9. The xenografts were moderately differentiated tubular adenocarcinoma. | [23] |
| S2-013 | Isolated from SUIT-2 | Clone S2-013 grew as an epithelial monolayer in a confluent state, the CEA and CA19-9 production levels were high, and the cells developed into a well-differentiated tubular adenocarcinoma. | [27] |
| MIA PaCa-2 | Pancreatic tissue of a patient with undifferentiated carcinoma | The cells have a doubling time of 40 h. Chromosomal counts of MIA PaCa-2 revealed a highly aneuploid pattern with the number of chromosomes ranging between 58 and 71. The cells contained measurable amounts of CEA, secreted a plasminogen activator, and were sensitive to l-asparaginase. | [38] |
| ASPC-1 | Derived from the ascites of a patient with cancer of the pancreas | The cells express PCAA and CEA. PCAA was different from CEA in its immunologic reactivity and distribution in its homologous tumoral tissues; PCAA was predominantly associated with the proliferative phase of the malignant epithelium. | [62] |
| Capan-2 | Pancreatic cancer tissue from patients with ductal carcinoma of the pancreatic head | The neoplastic cells showed characteristics of ductal epithelium, and the doubling time was 96 h. Chromosome studies showed a hypotetraploid pattern, and the cells exhibit a distinct enzyme phenotypic profile. In the xenografts, the tumor grew as a well-differentiated adenocarcinoma. | [70] |
| Panc02 | Ductal adenocarcinoma induced in mouse pancreatic tissue | The cells were obtained from a Grade II tumor. After transplantation back into mice, the tumor retained a well-differentiated histological appearance (a Grade III tumor appearance), and it was a solid tumor, which allowed for effective evaluation of metastasis rate (gross metastases were seen in the lungs of >70% of all tumor deaths). | [85] |
| PANC-1 | Human pancreatic carcinoma of ductal cells | The PANC-1-cell line has a doubling time of 52 h. The G6PD activity was of the slow mobility or B type, and chromosome studies showed a modal number of 63 with three distinct marker chromosomes and a small ring chromosome. Xenografts were progressively growing anaplastic carcinomas. | [88] |
- Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; G6PD, glucose-6-phosphate dehydrogenase; PCAA, pancreas cancer-associated antigen.
4 CONCLUSIONS AND FUTURE PERSPECTIVES
As one of the most deadly tumor types of the digestive system, PC has extremely high malignancy, limited treatment options, and poor prognosis. Many scientists have made unremitting efforts to further understand the mechanisms of the occurrence and development of PC, as well as explore more effective treatment options. The establishment of PC models is of great significance for preclinical research. Models that better match the biology of human PC are continually being developed, and many potential therapeutic targets and promising drugs have been validated using these models. However, there are still many difficulties to address. It is necessary to further study the molecular mechanisms of the identified therapeutic targets and the possible benefits/side effects of the drugs being examined for potential clinical use. In the future, richer treatment options will ultimately improve the prognosis of PC patients.
AUTHOR CONTRIBUTIONS
Weizheng Wu: Writing – original draft (equal); Writing – review and editing (equal). Kunming Wen: Methodology (equal); Supervision (equal). Yuxin Zhong: Conceptualization (lead); Supervision (lead).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.




