Inetetamab combined with sirolimus and chemotherapy for the treatment of HER2-positive metastatic breast cancer patients with abnormal activation of the PI3K/Akt/mTOR pathway after trastuzumab treatment
Qiao Li and Dan Lv contributed equally to this study.
Abstract
Background
We explored the efficacy and safety of inetetamab combined with sirolimus and chemotherapy for the treatment of human epidermal factor receptor 2 (HER2)-positive metastatic breast cancer patients with abnormal activation of the PI3K/Akt/mTOR (PAM) pathway after trastuzumab treatment.
Methods
For this prospective multicenter clinical study, HER2-positive metastatic breast cancer patients with PAM pathway mutations confirmed by histology or peripheral blood genetic testing were enrolled from July 2021 to September 2022. Patients were randomly assigned to a trial or control group. The patients in the trial group received inetetamab combined with sirolimus and chemotherapy, while the control group patients received pyrotinib and chemotherapy. The RECIST v1.1 standard was used to evaluate efficacy. Descriptive statistics were used to summarize the clinicopathological features, and the Kaplan–Meier method was used to generate survival curves. The log-rank test was used to compare progression-free survival (PFS) between the two groups.
Results
A total of 59 HER2-positive metastatic breast cancer patients with abnormal activation of the PAM pathway were included, of which 37 received inetetamab combined with sirolimus and chemotherapy treatment and 22 received pyrotinib and chemotherapy treatment. The median PFS was 4.64 months in the inetetamab group and 5.69 months in the pyrotinib group, with no statistically significant difference (p = 0.507). The objective response rates were 27.3% for the inetetamab group and 29.4% for the pyrotinib group. The safety assessment indicated that the adverse event (AE) incidences were 86.1% (31/36) in the inetetamab group and 78.9 (15/19) in the pyrotinib group, with 9 (25%) and four (21.1%) Grade 3/4 AEs in the inetetamab and pyrotinib groups, respectively.
Conclusions
For metastatic HER2-positive breast cancer patients with abnormal PAM pathway activation and previous trastuzumab treatment, the combination of inetetamab with sirolimus and chemotherapy is equivalent to the combination of pyrotinib and chemotherapy. Therefore, this regimen could be a treatment option for PAM pathway-activated metastatic HER2-positive breast cancer patients.
Abbreviations
-
- ADCC
-
- antibody-dependent cellular cytotoxicity
-
- CBR
-
- clinical benefit rate
-
- CR
-
- complete response
-
- CT
-
- computed tomography
-
- DCR
-
- disease control rate
-
- DOR
-
- duration of remission
-
- ECT
-
- emission computed tomography
-
- HER2
-
- human epidermal factor receptor 2
-
- MRI
-
- magnetic resonance imaging
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PAM
-
- PI3K/Akt/mTOR
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PI3K
-
- phosphatidylinositol-3-kinase
-
- PR
-
- partial response
-
- SD
-
- stable disease
1 INTRODUCTION
In 2020, breast cancer was the most commonly diagnosed cancer among women worldwide, with 2.26 million new cases reported. Breast cancer is one of the most common malignant tumors in women globally and the main type threatening the health of Chinese women [1]. Approximately 15%–20% of breast cancer patients have human epidermal growth factor receptor-2 (HER2) gene overexpression or amplification, which usually indicates a poor prognosis with worse progression-free survival (PFS) and overall survival (OS) [2]. Trastuzumab is the most widely used anti-HER2 monoclonal antibody drug. In recent decades, trastuzumab has significantly improved the prognosis of patients with HER2-positive breast cancer. However, 70% of advanced breast cancer patients still experience disease progression after receiving trastuzumab as a first-line treatment [3]. Studies have shown that continuous inhibition of the HER2 pathway can yield survival benefits for patients with HER2-positive metastatic breast cancer [4]. However, resistance remains the main issue hindering further improvements in the efficacy of anti-HER2 treatment strategies.
Studies have found that abnormal activation of the phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is associated with resistance to trastuzumab and small molecular tyrosine kinase inhibitors [3, 5, 6]. The prognosis of patients with mutations in this pathway is poor. Overcoming aberrant PI3K/Akt/mTOR (PAM) signaling is expected to enable patients to continue to benefit from anti-HER2 treatment methods [7]. The BOLERO-3 clinical study confirmed that treatment with the mTOR inhibitor everolimus combined with trastuzumab and vinorelbine demonstrated superior efficacy compared with the control treatment in patients with metastatic HER2-positive breast cancer after trastuzumab treatment failure. Everolimus combined with trastuzumab and vinorelbine further reduced the risk of disease recurrence by 22%, with PFS being extended from 5.8 to 7.0 months [8]. However, many adverse reactions and poor tolerance associated with the clinical application of everolimus have been reported by patients. This led to the discontinuation, dose reduction, or even termination of treatment for a high proportion of patients, thus affecting the treatment effect and reducing patient quality of life. Sirolimus is a specific mTOR antagonist that targets the PAM pathway and blocks its downstream signaling. Results of both preclinical and clinical studies have shown that sirolimus treatment can inhibit tumor proliferation and angiogenesis, has fewer adverse reactions, and is safer and more tolerable than everolimus [9].
Inetetamab is an innovative anti-HER2 monoclonal antibody that has a modified and optimized Fc region, with a stronger antibody-dependent cellular cytotoxicity (ADCC) effect. Preclinical studies have shown that inetetamab and trastuzumab display comparable HER2 antigen binding ability and affinity. Additionally, the two antibodies have similar tumor cell proliferation inhibition abilities, protein structures, and thermal stability. The ADCC effect of inetetamab is 1.1 times stronger than that of trastuzumab [10].
In this study, we explored the use of inetetamab combined with sirolimus to simultaneously inhibit HER2 and the PAM pathway in patients with abnormal activation of this pathway and disease progression after trastuzumab treatment. We investigated if this treatment approach could help redevelop tumor sensitivity to HER2 monotherapy, which would allow these patients to continue to benefit from anti-HER2 treatment. Overall, this strategy would greatly improve the treatment effect and prognosis of such patients.
2 METHODS
2.1 Research subjects and experimental design
This was a prospective, multicenter, randomized, controlled clinical study. The purpose of this study was to compare the efficacy between inetetamab and sirlimus group (trial group) and pyrotinib group (control group) in HER2-positive metastatic breast cancer patients with abnormal activation of the PAM pathway and disease progression after trastuzumab treatment. The study was approved by the Institutional Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (approval number: NCC2445).
Fifty-nine patients from a total of 21 centers, including the Cancer Hospital of the Chinese Academy of Medical Sciences, were enrolled in this study between July 2021 and September 2022. The inclusion criteria were as follows: women >18 years of age; HER2 overexpression and +++ detected by immunohistochemistry (IHC) or positive fluorescence in situ hybridization (FISH) test; histologically or cytologically confirmed invasive breast cancer, with local recurrence or radiological evidence of metastatic lesions; HER2-positive breast cancer patients with disease progression after treatment with trastuzumab (including trastuzumab, trastuzumab combined with pertuzumab, and T-DM1); patients with PAM pathway-related gene mutations, as determined by genetic testing; Eastern Cooperative Oncology Group Performance Status (ECOG PS) score ≤2, expected survival ≥6 months, and able to be followed up; clear measurable and/or evaluable lesions according to RECIST 1.1 criteria; normal cardiopulmonary function, with left ventricular ejection fraction (LVEF) ≥ 50% within 4 weeks before starting treatment; sufficient liver function and bone marrow function, generally normal coagulation function, and no history of serious heart, kidney, and other important organ or endocrine system diseases; negative pregnancy test for women of childbearing age and voluntarily use of effective and reliable contraception; and patients who voluntarily signed an informed consent form. The exclusion criteria were as follows: previous use of mTOR inhibitors; previous use of pyrotinib in the advanced first-line treatment stage (previous use of lapatinib was allowed); chronic corticosteroid use for >3 months or within 4 weeks plus immunosuppressant use or radiation therapy for bone marrow replacement of 25% or more; evidence of symptomatic central nervous system metastasis or leptomeningeal disease; LVEF < 50% of cardiac function; clinical manifestations of significant arrhythmias, myocardial ischemia, severe atrioventricular block, cardiac dysfunction, or severe valvular heart disease; gastrointestinal dysfunction or gastrointestinal diseases (including active ulcers); chronic liver disease; and other factors considered by the investigators to be inappropriate for participation in this trial, such as any other medicines, social factors, or psychological factors that may affect their safety or compliance with study procedures.
PAM pathway mutations were detected as follows: Blood was collected in a cell-free DNA (cfDNA)-stabilizing collection tube, and then plasma was separated within 5 days of blood draw. Plasma cfDNA was extracted with the QIAamp Circulating Nucleic Acid Kit (Qiagen) following the manufacturer's instructions. PIK3CA mutation detection was performed using digital PCR with a custom panel (Mission Medical Technologies [Ningbo] Co. Ltd.) covering the six most frequently observed mutations in Chinese patients (see Table S1) on a Bio Digital Qing digital PCR system (Shanghai Turtle Technologies).
2.2 Specific medication regimens
In the inetetamab and sirolimus group, the patients were given the first dose of inetetamab at 8 mg/kg by intravenous infusion over 90 min, followed by a maintenance dose (6 mg/kg) every 3 weeks by intravenous infusion over 30–90 min until disease progression or other termination criteria were met; patients were also administered sirolimus (2 mg orally) once a day. In the pyrotinib group, the patients were given pyrotinib (400 mg orally) once a day. The chemotherapeutic drugs for the patients in the two groups were not limited, with the physicians selecting such drugs based on the conditions of the patients. All patients included in this study voluntarily signed an informed consent form. The enrolled patients received the corresponding treatments until tumor progression, intolerable adverse reactions, or requested to withdraw from the study for personal reasons.
2.3 Evaluation of efficacy
Efficacy was evaluated according to the RECIST v1.1 standard. At baseline, patients underwent computed tomography (CT) or magnetic resonance imaging (MRI) scans, and patients with bone metastasis received whole-body bone emission computed tomography (ECT) scans to evaluate tumor status. Efficacy assessments were performed every two treatment cycles (21 days as one treatment cycle), and safety assessments were performed once every treatment cycle (21 days) until progressive disease (PD).
2.4 Safety assessment
All adverse events were rated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0 [11].
2.5 Statistical analysis
All statistical analyses were performed using SPSS 22.0 statistical analysis software (IBM Corp.) or GraphPad Prism 8.0 (GraphPad Software). Descriptive statistics were used to summarize the clinicopathological features, such as the median and percentage of patients. Categorical variables were compared using the chi-square test or Fisher's exact test. PFS was defined as the duration from when patients first received a study drug to the day of disease progression or death. The objective response rate (ORR) was defined as the ratio of patients with complete response (CR) and partial response (PR) among the total population. The disease control rate (DCR) was defined as the ratio of patients with CR, PR, and stable disease (SD) among the total population. Survival curves were created using the Kaplan–Meier (KM) method, and the log-rank test was used to compare PFS between the inetetamab and pyrotinib groups. Cox regression models were used to compare the relationships between the clinical and pathological factors and PFS between the inetetamab and pyrotinib groups. The median follow-up time was calculated by the reverse KM method. In this study, p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Clinicopathological characteristics of patients and specimens
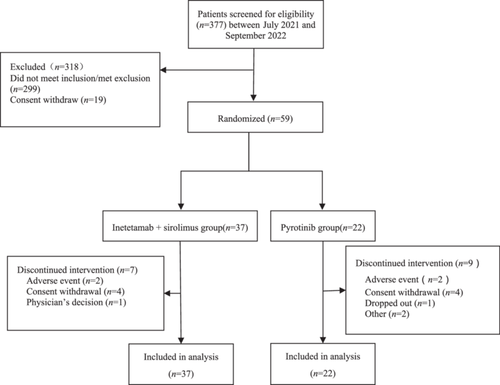
A total of 59 HER2-positive metastatic breast cancer patients with abnormal activation of the PAM pathway confirmed by gene detection were included. Figure 1 summarizes the study flow. The most frequent mutations were p.H1047R (37.9%), followed by p.E545K (15.6%), p.E542K (8.6%), p.H1047L (5.5%), p.N345K (5.5%), and p.E726K (3.0%). Among them, 37 patients received inetetamab and 22 patients received pyrotinib. The patients were between 31 and 74 years of age, with a median age of 53 years. Fifty-one patients were younger than 65 years (86.44%), and eight patients were older than 65 years (13.56%). Thirty patients (50.8%) were HR-positive/HER2-positive, and 29 patients (49.2%) were HR-negative/HER2-positive. After breast cancer recurrence and metastasis, 46 patients (78.0%) developed visceral metastasis [26 patients (70.3%) in the trial group and 20 patients (90.9%) in the control group], 31 (52.5%) of whom developed lung metastasis and 30 (50.8%) of whom developed liver metastasis. The average number of previous treatment lines was 2.6 in the inetetamab group and 1.4 in the pyrotinib group (p = 0.016). Six patients (16.2%) in the inetetamab group and seven patients (31.8%) in the pyrotinib group had never received chemotherapy after recurrence and metastasis. Four patients (10.8%) in the inetetamab group and eight patients (36.4%) in the pyrotinib group had received first-line treatment. For chemotherapy, 12 patients (32.4%) in the inetetamab group and three patients (13.6%) in the pyrotinib group had received second-line chemotherapy, while 10 patients (27.0%) in the inetetamab group and two patients (9.1%) in the pyrotinib group had received more lines of chemotherapy. The differences between groups were statistically significant. The baseline characteristics of all the included patients are shown in Table 1.
| Variable | Total population (n = 59) | Inetetamab + sirolimus group (n = 37) | Pyrotinib group (n = 22) | p |
|---|---|---|---|---|
| Age | 53.9 ± 8.8 | 53.6 ± 9.9 | 54.3 ± 6.7 | 0.605 |
| HR status | 0.086 | |||
| ER/PR positive | 30 (50.8) | 22 (59.5) | 8 (36.4) | |
| ER/PR negative | 29 (49.2) | 15 (40.5) | 14 (63.6) | |
| Number of previous treatment lines | 2.1 | 2.6 | 1.4 | 0.016 |
| 0 | 13 (22.0) | 6 (16.2) | 7 (31.8) | |
| 1 | 12 (20.3) | 4 (10.8) | 8 (36.4) | |
| 2 | 15 (25.4) | 12 (32.4) | 3 (13.6) | |
| 3 | 7 (11.9) | 5 (13.5) | 2 (9.1) | |
| 4 | 4 (6.8) | 3 (8.1) | 1 (4.5) | |
| >4 | 8 (13.6) | 7 (18.9) | 1 (4.5) | |
| Visceral metastasis | 46 (78.0) | 26 (70.3) | 20 (90.9) | 0.341 |
| Lung metastasis | 31 (52.5) | 18 (48.6) | 13 (59.1) | 0.701 |
| Liver metastasis | 30 (50.8) | 17 (45.9) | 13 (59.1) | |
| Previous use of targeted drugs | 56 (94.9) | 36 (97.3) | 20 (90.9) | |
| Trastuzumab | 53 (89.8) | 33 (89.2) | 20 (90.9) | |
| Pertuzumab | 18 (30.5) | 7 (18.9) | 11 (50.0) | |
| Trastuzumab + Pertuzumab | 3 (5.1) | 1 (2.7) | 2 (9.1) | |
| Pyrotinib | 18 (30.5) | 16 (43.2) | 2 (9.1) | |
| Lapatinib | 10 (16.9) | 7 (18.9) | 3 (13.6) | |
| Other | 35 (59.3) | 27 (73.0) | 8 (36.4) |
There was no significant difference observed in age, HR status, or the presence of visceral metastases between the inetetamab and pyrotinib groups (p > 0.05).
The median follow-up duration was 5.33 months; the median follow-up duration was 5.53 months in the inetetamab group and 4.53 months in the pyrotinib group.
3.2 Efficacy
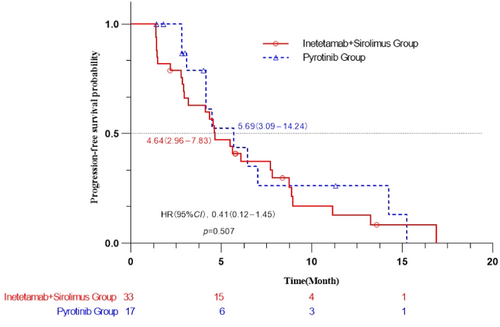
The best efficacy achieved by each patient during the study was recorded. The best efficacy could not be evaluated for ten patients, six because of withdrawal of informed consent and four because of intolerable toxicity. For the efficacy analysis, 33 patients were included in the inetetamab group, and 17 patients were included in the pyrotinib group. The median PFS of inetetamab group patients was 4.64 (2.96–7.83) months, which was similar to that of pyrotinib group patients (5.69 [3.09–14.24] months). The difference between the two groups was not significant (p = 0.507; hazard ratio [HR] = 0.41; 95% confidence interval [CI] = 0.12–1.45) (Figure 2). The Cox multivariate analysis results showed that among the subjects with visceral metastasis (liver metastasis and lung metastasis), the median PFS and 95% CI were 4.34 (2.80–5.63) months for the inetetamab group and 4.47 (3.09–14.24) months for the pyrotinib group. This difference was also not significant (p = 0.327; HR = 0.40; 95% CI = 0.08–2.00). Among the subjects with fewer than two previous treatment lines, the median PFS and 95% CI were 4.54 (1.48–8.88) months in the inetetamab group and 4.14 (2.83–NA) months in the pyrotinib group, with no significant difference (p = 0.686; HR = 0.23; 95% CI = 0.01–3.66). Among the subjects with two or more previous treatment lines, the median PFS and 95% CI were 4.34 (1.41–7.73) months in the inetetamab group and 2.96 (2.83–NA) months in the pyrotinib group, with no significant difference (p = 0.352; HR = 0.63; 95% CI = 0.05–8.43). Among the subjects who were previously treated with trastuzumab, the median PFS and 95% CI were 4.61 (2.93–7.83) months in the inetetamab group and 5.69 (3.09–14.24) months in the pyrotinib group, with no significant difference (p = 0.596; HR = 0.41; 95% CI = 0.12–1.45).
The overall evaluation of best efficacy was performed based on RECIST 1.1. In the inetetamab group, nine patients achieved PR, 16 patients achieved SD, and seven patients had PD. In the pyrotinib group, five patients achieved PR, and 12 patients achieved SD. Among the patients who received inetetamab combined with sirolimus, nine (27.3%) patients experienced disease remission, while the disease was controlled in 25 patients (75.8%). Among the patients in the pyrotinib group, five patients (29.4%) achieved disease remission, while the disease was controlled in all 17 patients (100%). The ORR and 95% CIs were 27.3% (13.75–46.75) for the inetetamab group and 29.4% (10.31–55.96) for the pyrotinib group, with no significant difference (p = 1.000). The clinical benefit rate (CBR) and 95% CIs were 33.3% (17.96–51.83) for the inetetamab group and 41.2% (18.44–67.08) for the pyrotinib group, with no significant difference (p = 0.584). The median duration of remission (DOR) and 95% CI were 7.57 (1.48–NA) months for the inetetamab group and 3.03 (1.55–NA) months for the pyrotinib group, with no significant difference (p = 0.726) (Table 2).
| Variable | Inetetamab + sirolimus group (n = 33, %) | Pyrotinib group (n = 17, %) | p |
|---|---|---|---|
| Overall assessment | |||
| CR | 0 (0) | 0 (0) | |
| PR | 9 (27.3) | 5 (29.4) | |
| SD | 16 (48.5) | 12 (70.6) | |
| PD | 7 (21.2) | 0 (0) | |
| Could not assess | 1 (3.0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | |
| Objective remission rate (%) | 9 (27.3) | 5 (29.4) | 1.000 |
| 95% confidence interval (CI) | (13.8, 46.8) | (10.3, 56.0) | |
| Clinical benefit rate (%) | 11 (33.3) | 7 (41.2) | 0.584 |
| 95% CI | (18.0, 51.8) | (18.4, 67.1) |
3.3 Safety
The overall incidence of treatment-related adverse events was 86.1 (31/36) in the inetetamab group and 78.9% (15/19) in the pyrotinib group. The incidence of grade 3 and above adverse events was 25.0% (9/36) in the inetetamab group and 21.1% (4/19) in the pyrotinib group. Among them, increased aminotransferase (41.7% vs. 21.1%), reduced neutrophil count (33.3% vs. 31.6%), anemia (33.3% vs. 26.3%), and reduced white blood cell count (27.8% vs. 26.3%) were the most commonly reported adverse events. A total of nine patients (25.0%) in the inetetamab group and four patients (21.1%) in the pyrotinib group had treatment-related grade 3 and above adverse events. The adverse events with a frequency of ≥10% were diarrhea (13.9% vs. 42.1%), nausea/vomiting (13.9% vs. 26.3%), oral mucositis (22.8% vs. 15.8%), increased bilirubin (2.8% vs. 10.5%), increased blood glucose (13.9% vs. 10.5%), hyperlipidemia (11.1% vs. 5.2%), hypertriglyceridemia (19.4% vs. 10.5%), and hand-foot syndrome (0% vs. 15.8%) (Table 3).
| Inetetamab + sirolimus group (n = 36, %) | Pyrotinib group (n = 19, %) | |||
|---|---|---|---|---|
| Treatment-related adverse events | Any level | Grade 3/4 | Any level | Grade 3/4 |
| Any event | 31 (86.1) | 9 (25.0) | 15 (78.9) | 4 (21.1) |
| Leukopenia | 10 (27.8) | 0 (0) | 5 (26.3) | 2 (10.5) |
| Neutropenia | 12 (33.3) | 3 (8.3) | 6 (31.6) | 0 (0) |
| Thrombocytopenia | 3 (8.3) | 0 (0) | 1 (5.3) | 0 (0) |
| Anemia | 12 (33.3) | 2 (5.6) | 5 (26.3) | 0 (0) |
| Diarrhea | 5 (13.9) | 1 (2.8) | 8 (42.1) | 1 (5.3) |
| Nausea/vomiting | 5 (13.9) | 0 (0) | 5 (26.3) | 0 (0) |
| Elevated aminotransferases | 15 (41.7) | 2 (5.6) | 4 (21.1) | 0 (0) |
| Elevated bilirubin | 1 (2.8) | 0 (0) | 2 (10.5) | 0 (0) |
| Elevated creatinine | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) |
| Elevated blood glucose | 5 (13.9) | 0 (0) | 2 (10.5) | 0 (0) |
| Hyperlipidemia | 4 (11.1) | 0 (0) | 1 (5.3) | 0 (0) |
| Hypertriglyceridemia | 7 (19.4) | 1 (2.8) | 2 (10.5) | 0 (0) |
| Oral mucositis | 8 (22.2) | 1 (2.8) | 3 (15.8) | 0 (0) |
| Sinus tachycardia | 1 (2.8) | 0 (0) | 1 (5.3) | 0 (0) |
| Hand-foot syndrome | 0 (0) | 0 (0) | 3 (15.8) | 1 (5.3) |
| Neurotoxicity | 2 (5.6) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial pneumonia | 1 (2.8) | 1 (2.8) | 0 (0) | 0 (0) |
| Venous thrombosis | 1 (2.8) | 0 (0) | 0 (0) | 0 (0) |
| Cardiac dysfunction | 1 (2.8) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 1 (2.8) | 0 (0) | 1 (5.3) | 0 (0) |
In the inetetamab + sirolimus group, three patients (8.1%) were treated with a reduced sirolimus dose because of grade 3 and above adverse events, two patients (5.4%) were permanently discontinued because of intolerance, and two patients (5.4%) were treated with a reduced dose of chemotherapy drugs. In the pyrotinib group, seven patients (33.3%) received a reduction of pyrotinib because of grade 3 and above adverse events, while three patients (14.3%) received a reduced dose of chemotherapy drugs.
4 DISCUSSION
HER2 protein phosphorylation can lead to downstream activation of the PAM pathway, which is one of the causes of trastuzumab drug resistance [12]. Therapeutic strategies targeting this pathway have been shown to alleviate the resistance to anti-HER2 therapy and enhance the treatment effect. A phase I/II clinical study showed that the combination of the PI3K inhibitor pilaralisib with trastuzumab and paclitaxel had certain clinical efficacy [13]. The BOLERO-3 study compared the mTOR inhibitor everolimus with placebo combined with trastuzumab and vinorelbine for the treatment of HER2-positive metastatic breast cancer. Compared with placebo combined with trastuzumab and vinorelbine, everolimus treatment reduced the risk of disease progression by 22% and prolonged PFS [8]. The BOLERO-1 study evaluated the effect of everolimus in patients with HER2-positive metastatic breast cancer after drug resistance to trastuzumab. Everolimus treatment did not yield survival benefits to patients beyond those of the placebo [14]. Subsequent joint exploratory analysis of the BOLERO-1 and BOLERO-3 studies showed that patients with a PIK3CA mutation, PTEN deletion, and overactivation of the PAM pathway may gain PFS benefits from everolimus treatment [15]. However, further investigation is needed to determine whether the simultaneous administration of PI3K inhibitors and accurate targeted therapy against HER2 yields further clinical benefits to HER2-positive metastatic breast cancer patients with abnormal PAM pathway activation.
Inetetamab is an innovative HER2-targeting monoclonal antibody with a modified and optimized Fc region. In contrast to trastuzumab and pertuzumab, the two amino acids of the inetetamab Fc domain have been mutated to exhibit a stronger ADCC effect [16]. Retrospective studies have shown that sirolimus, an mTOR inhibitor, combined with endocrine therapy in patients with hormone receptor-positive breast cancer can further improve the median PFS [9]. A few phase II studies have also demonstrated that sirolimus combined with trastuzumab can reverse trastuzumab resistance and yield treatment benefits in patients with HER2-positive metastatic breast cancer [17]. This study focused on HER2-positive metastatic breast cancer patients with abnormal PAM pathway activation that had progressed after trastuzumab treatment. Clinical efficacy and adverse reactions were observed after patients received inetetamab combined with sirolimus and chemotherapy or pyrotinib combined with chemotherapy.
In this study, the PFS results were comparable between patients in the inetetamab and pyrotinib groups. While 72.9% of patients in the inetetamab group received two or more treatments, only 31.7% of patients received two or more treatment regimens in the pyrotinib group. The median number of treatment lines for the inetetamab group was 2.6 lines, which is more than the median number of treatment lines for the pyrotinib group.
The disease remission rate of the pyrotinib group was 29.4%, which was slightly higher than that of the inetetamab group (27.3%). For the DCR, all 17 patients (100%) of the pyrotinib group achieved stability. This was higher than that of the inetetamab group (75.8%), but the difference was not statistically significant. For treatment tolerability and safety, 31 patients (86.1%) in the inetetamab group had various degrees of adverse events, with the most common ones being hematologic toxicity, increased aminotransferases, oral mucositis, hyperglycemia, and hypertriglyceridemia. Varying degrees of adverse events were also observed in the pyrotinib group, with the most common being diarrhea, followed by hematologic toxicity, nausea/vomiting, increased aminotransferases, oral mucositis, and hand-foot syndrome. In the pyrotinib group, 42.1% of patients experienced varying degrees of diarrhea, and 26.3% of patients exhibited symptoms of nausea and vomiting. No death-related serious adverse events were observed in either group. The safety and tolerability results were acceptable.
Oral mucositis is the most common adverse reaction in patients treated with mTOR inhibitors. A previous study showed that the incidence of oral mucositis with everolimus treatment was 8% [18]. However, the incidence of oral mucositis in this study was only 2.8%, lower than that observed with everolimus. The incidence of interstitial pneumonia was also lower than that reported for everolimus [18, 19]. In the inetetamab group, one patient (2.8%) had grade III interstitial pneumonia. This was considered to be related to sirolimus administration, and the patient recovered after discontinuing symptomatic treatment. In the inetetamab group, only 13.9% of patients had symptoms of diarrhea, and 13.9% of patients experienced nausea and vomiting. In comparison, the inetetamab group was superior to the pyrotinib group in terms of perceived toxicity.
This study has limitations. First, the follow-up time was short, and OS was not fully evaluated; further follow-up is needed. Second, no limit was set for the number of previous treatment lines for patient inclusion, which resulted in an unbalanced number of previous chemotherapy lines between the two groups. Third, because this was a multicenter study, the HER2 status of patients at other research centers was not confirmed by our center. Fourth, the sample size in the present study was relatively small; future similar studies should have larger sample sizes.
5 CONCLUSIONS
In this study, the efficacy of inetetamab combined with sirolimus and chemotherapy was comparable to that of the pyrotinib chemotherapy combination regimen in trastuzumab-pretreated metastatic HER2-positive breast cancer patients with aberrant activation of the PAM pathway. The two regimens displayed different adverse reaction profiles, with the inetetamab group being superior to the pyrotinib group in perceived toxicity. Therefore, inetetamab combined with sirolimus and chemotherapy can be used as a treatment option for pretreated PAM pathway-activated HER2-positive breast cancer patients.
AUTHOR CONTRIBUTIONS
Qiao Li: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing—original draft (equal). Dan Lv: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal). Xiaoying Sun: Data curation (equal); project administration (equal). Mengyuan Wang: Data curation (equal); project administration (equal). Li Cai: Data curation (equal); project administration (equal). Feng Liu: Data curation (equal); project administration (equal). Chenghui Li: Data curation (equal); project administration (equal). Jiuda Zhao: Data curation (equal); project administration (equal). Jing Sun: Data curation (equal); project administration (equal). Yehui Shi: Data curation (equal); project administration (equal). Fei Ma: Conceptualization (lead); formal analysis (equal); funding acquisition (equal); supervision (lead); writing—original draft (equal); writing—review and editing (lead).
ACKNOWLEDGMENTS
None.
CONFLICTS OF INTEREST STATEMENT
Professors Jiuda Zhao and Fei Ma are members of the Cancer Innovation Editorial Board. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Institutional Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (approval number: NCC2445).
INFORMED CONSENT
All patients provided written informed consent at the time of entering this study.
Open Research
DATA AVAILABILITY STATEMENT
Datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.




