Clinical practice guidelines for full-cycle standardized management of bone health in breast cancer patients
Correspondence Fei Ma, Department of Medical Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Email: [email protected]
Abstract
Bone health management for breast cancer spans the entire cycle of patient care, including the prevention and treatment of bone loss caused by early breast cancer treatment, the adjuvant application of bone-modifying agents to improve prognosis, and the diagnosis and treatment of advanced bone metastases. Making good bone health management means formulating appropriate treatment strategies and dealing with adverse drug reactions, and will help to improve patients' quality of life and survival rates. The Breast Cancer Expert Committee of the National Cancer Center for Quality Control organized relevant experts to conduct an in-depth discussion on the full-cycle management of breast cancer bone health based on evidence-based medicine, and put forward reasonable suggestions to guide clinicians to better deal with health issues in bone health clinics.
Abbreviations
-
- AI
-
- aromatase inhibitor
-
- BMD
-
- bone mineral density
-
- BMFS
-
- bone metastasis-free survival
-
- CTIBL
-
- cancer treatment-induced bone loss
-
- DFS
-
- disease-free survival
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HR
-
- hormone receptor
-
- MRONJ
-
- medication-related osteonecrosis of the jaw
-
- OFS
-
- Ovarian function suppression
-
- OS
-
- overall survival
-
- SRE
-
- skeletal-related events
1 INTRODUCTION
Breast cancer is the most common malignant tumor in the world [1]. With the popularization of early breast cancer screening and the improvement of comprehensive treatment, the 5-year survival rate of breast cancer is increasing year by year. Data from the National Cancer Center show that the 5-year survival rate of breast cancer patients in China exceeds 83% [2]. As the survival period of breast cancer patients prolongs, the full-cycle standardized management of bone health in breast cancer patients has become increasingly important and imperative.
Standardized management of bone health in the full cycle of breast cancer includes bone health management for patients with early breast cancer, bone health management for patients with advanced breast cancer, and safety management of bone-modifying agents. Bone health management for patients with early breast cancer mainly includes the prevention of bone loss and bone metastasis, as well as the adjuvant application of bone-modifying agents to improve survival benefits. Breast cancer patients often suffer from bone loss after receiving chemotherapy, aromatase inhibitor (AI) treatment, ovarian function suppression (OFS), and so forth, which is called cancer treatment-induced bone loss (CTIBL). Bone loss may be accompanied by abnormal bone metabolism, which will significantly increase the risk of weight-bearing bone fracture and affect the patient's survival and quality of life. Therefore, preventing and treating bone loss will bring certain benefits to patients.
The main goals of bone health management in patients with advanced breast cancer bone metastases are to prevent and treat skeletal-related events (SRE) and improve the quality of life [3]. Bone health management needs to emphasize the use of bone-modifying agents. Bone-modifying agents may cause adverse reactions in the short term, such as flu-like symptoms, hypocalcemia, and so forth. As the medication time is prolonged, some patients may suffer from renal function damage, osteonecrosis of the jaw, and so forth, thus causing adverse effects on patient's health. Therefore, the rational application of bone-modifying agents and the correct identification and management of adverse drug reactions are the keys and run through the entire bone health management.
Since there is no systematic management plan and standardized diagnosis and treatment recommendations for breast cancer patients' bone health, to further improve the prognosis of breast cancer patients and improve their survival rate and quality of life, experts in the field of breast cancer as well as in radiotherapy, orthopedics and other related disciplines in China, based on the latest domestic and foreign guidelines and evidence-based medicine, formulate the “Clinical practice guidelines for full-cycle standardized management of bone health in breast cancer patients” to provide a reference basis for the management of bone health and standardized diagnosis and treatment for breast cancer patients.
2 BONE HEALTH MANAGEMENT FOR EARLY BREAST CANCER PATIENTS
Bone health management for patients with early breast cancer includes: (1) Prevention and treatment of CTIBL, and (2) prevention of bone metastasis and potential recurrence risk to improve overall survival rate.
2.1 Prevention and treatment of CTIBL in early breast cancer patients
2.1.1 Background
Of breast cancer patients, 80% are in the early stages, so adjuvant therapy is widely used and crucial for them. Chemotherapy and endocrine therapy play an important role in the treatment process, and the bone loss caused by corresponding treatments should be taken seriously. Therefore, the prevention and treatment of bone loss is particularly important in the bone health management of early breast cancer patients.
Bone growth begins before birth, and by age 30, female bones reach more than 95% of their maximum strength and density. Thereafter, bones change only very slightly until menopause, when estrogen levels drop rapidly, causing massive bone loss. Estrogen has an important protective effect on bone mass. Estrogen increases osteoblast function, induces osteoclast apoptosis, and inhibits bone resorption. The rapid decrease in estrogen levels in postmenopausal women leads to accelerated osteoblast differentiation, promotes osteoclast formation and enhances their activity, and accelerates bone loss. Especially within 10 years after menopause, bone loss due to estrogen deficiency is 2%–3% per year [4, 5]. This causes bone mineral density (BMD) to decrease year by year, making patients prone to osteoporosis and fractures [6]. Bone loss is further accelerated when patients receive antitumor treatments such as endocrine therapy and chemotherapy. Studies have shown that the spine bone loss rate of breast cancer patients treated with AI is 2.2%–2.6% per year, and the hip bone loss rate is 1.7%–2.2% [7, 8].
Bone loss leads to an increased risk of fractures, which in turn affects patient survival. The 12-month survival rate and 5-year survival rate for women after spinal fractures are 86.5% and 56.5%, respectively [4, 9]. Meanwhile, related treatments not only bring heavy financial burdens and psychological pressure to patients, but also bring significant economic burdens to society. Therefore, clinicians should routinely assess the bone health status of corresponding patients to prevent and treat CTIBL.
2.1.2 Factors affecting CTIBL
OFS therapy in premenopausal patients, AI therapy in postmenopausal patients, chemotherapy, ovarian radiotherapy, ovariectomy, and other debulking treatments can cause a significant decrease in estrogen levels in patients, resulting in bone loss. Among them, OFS and AI treatments are the most significant factors leading to bone loss. For premenopausal patients, OFS treatment can effectively reduce serum estrogen levels and bring the patient's estrogen levels to postmenopausal levels. For postmenopausal patients, AI treatment will further reduce the patient's endogenous estrogen levels, both of which will lead to accelerated bone loss and increased risk of fractures [10, 11]. In premenopausal breast cancer patients who received OFS for 2 years, lumbar spine BMD decreased by 10.5% from baseline and femoral neck BMD decreased by 6.4% [12]. For women who undergo early menopause before the age of 45, BMD declines at a faster rate, averaging 3%–4% per year [13]. Postmenopausal breast cancer patients treated with AI have a 17% increased risk of fracture compared with untreated patients [14]. Patients receiving extended endocrine therapy were 1.34 times more likely to experience a clinical fracture than patients who received no treatment or placebo alone [15].
In addition to factors related to tumor treatment, there are other factors that can affect a patient's bone health, such as poor lifestyle habits, various endocrine system diseases including hypogonadism, rheumatic immune diseases, digestive and renal disorders, and others that affect the absorption and metabolism of calcium and vitamin D. Drugs such as glucocorticoid and thyroid hormone overdose can cause bone loss [16, 17], and clinical treatment should pay attention to this.
2.1.3 Assessment of bone and bone metabolism
2.1.3.1 BMD detection
BMD is the main indicator to evaluate bone loss and osteoporosis in postmenopausal breast cancer patients. The common method for detecting BMD is dual-energy X-ray absorptiometry (DXA), whose main measurement site is the mid-shaft bones, including the orthotopic lumbar spine and the proximal femur (hip bone). If the measurement of the lumbar spine and proximal femur is limited, the distal 1/3 of the radius on the nondominant side can be selected as the measurement site [18].
2.1.3.2 Fracture risk assessment tool
In addition to BMD, the European Society for Medical Oncology (ESMO) Bone Health Guidelines and the American Society of Clinical Oncology (ASCO) guidelines recommend the World Health Organization Fracture Risk Assessment Tool (FRAX) (www.shef.ac.uk/FRAX/, and its app is also available) as an assessment tool to quantify the risk of osteoporosis in early breast cancer [19, 20]. The FRAX tool predicts the 10-year risk of incident osteoporotic fractures in healthy postmenopausal women based on age, sex, clinical risk factors, femoral neck BMD (T-score), and other factors. And this data is also applicable to Chinese postmenopausal women [21]. However, the FRAX tool does not include antitumor treatment as a specific risk factor, and its impact on bone loss in postmenopausal breast cancer patients may be underestimated. Clinicians should assess fracture risk by integrating this tool with disease and treatment.
2.1.3.3 Bone biochemical marker monitoring
Bone turnover biochemical markers are metabolic products of the bones themselves and can dynamically reflect the overall condition of the skeletal system. Measurement of biochemical markers of bone turnover can help determine the type of bone turnover and bone loss rate, assess fracture risk, understand disease progression, select interventions, and judge the efficacy of antiosteoporotic treatments. Currently commonly used bone formation markers include bone alkaline phosphatase (BALP), procollagen type I N-terminal propeptide (PINP), and osteocalcin, which can reflect the ability of bone formation. Bone resorption markers include N-terminal telopeptide of type I collagen (NTX), C-terminal telopeptide of type I collagen (CTX), C-terminal propeptide of type I procollagen (ICTP), and pyridinoline cross-linked peptides, and so forth, which can reflect the activity of osteoclasts and the degradation of type I collagen. When bisphosphonates are used to treat bone loss, these bone turnover indicators can be used to monitor treatment response [22]. Hospitals with the necessary conditions can monitor biochemical markers [21].
2.1.3.4 Bone loss and osteoporosis risk classification
Classification of fracture risk caused by CTIBL according to BMD, FRAX tool, and clinical factors are shown in Table 1.
| Risk classification | Risk classification influencing factors |
|---|---|
| Low risk | T-score ≥ −1.0 and unplanned or unused AI treatment. |
| Moderate risk | (1) T-score ≥ −1.0 and planned or ongoing AI therapy; (2) −2.5 < T-score < −1.0 and unplanned or unused AI. It can be determined if one of the above two conditions is met. |
| High risk |
For condition (3), it can be determined if any one of the above three conditions is met. |
2.1.4 Prevention and treatment of CTIBL in patients with early breast cancer
2.1.4.1 Prevention and control strategies
- a.
Low-risk patients: It is recommended to improve lifestyle and supplement calcium and vitamin D.
- b.
Moderate-risk patients: It is recommended to improve lifestyle, supplement calcium and vitamin D, and consider bone-modifying agents.
- c.
High-risk patients: It is recommended to improve lifestyle, supplement calcium, vitamin D, and bone-modifying agents.
The recommended clinical pathway for CTIBL prevention and treatment of early breast cancer is shown in Figure 1.
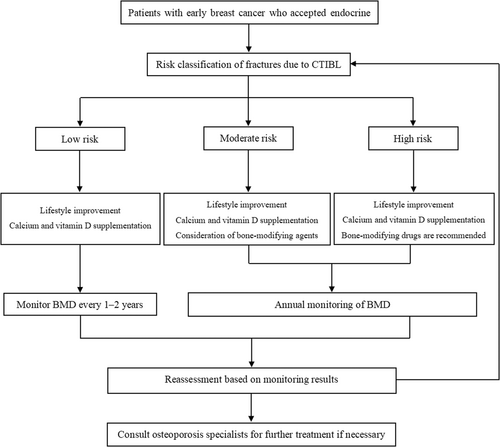
2.1.4.2 Specific contents of prevention and control
- a.
Improvement of lifestyle: All patients should improve their lifestyle, and lifestyle intervention is the basis for the prevention and treatment of CTIBL in patients with early breast cancer [21, 23].
-
Adjust dietary structure: It is recommended to eat calcium-rich foods, increase the intake of a variety of vegetables and fruits, choose whole grains or high-fiber foods, eat fish at least twice a week, and limit the intake of saturated and trans-unsaturated fatty acids, alcohol, cholesterol, and sugar, avoid excessive consumption of coffee and carbonated drinks.
-
Exercise: Do at least 150 min of moderate-intensity aerobic exercise every week, such as jogging, walking, swimming, cycling, dancing, and so forth. Postmenopausal women should perform muscle tone exercises at least twice a week, but be careful to prevent falls and physical collisions [21]. Increasing light exposure can promote the synthesis of vitamin D in the body.
-
Lose or maintain weight: Maintain or reduce weight through exercise, diet control, and behavioral training, so that the body mass index (BMI) is kept between 20 and 24 kg/m2, and the waist circumference is less than 80 cm.
-
Quit smoking and drinking: Since smoking increases the risk of osteoporosis, drinking alcohol can also affect a patient's bone density. Encourage patients to quit smoking, avoid second-hand smoke, and stop drinking alcohol.
-
- b.
Supplement calcium and vitamin D
-
Calcium: Adequate calcium helps reduce bone loss, promote bone mineralization, and maintain bone health. The International Osteoporosis Foundation recommends that postmenopausal women consume 1300 mg of elemental calcium per day [24]. The “Reference Intake of Dietary Nutrients for Chinese Residents” recommends that the recommended daily intake of elemental calcium for middle-aged and elderly people over 50 years old is 1000–1200 mg, and the maximum tolerable intake is 2000 mg. Nutritional surveys show that the daily dietary elemental calcium intake of Chinese residents is about 400 mg, so it is still necessary to supplement about 500–600 mg/day of elemental calcium. Calcium supplements are usually calcium carbonate and calcium citrate. Calcium carbonate has high calcium content, good absorption rate, and is easily soluble in gastric acid. Common adverse reactions include upper abdominal discomfort, constipation, hypercalcemia, hypercalciuria, and so forth. Calcium citrate contains relatively low calcium, has good water solubility, does not rely on gastric acid for dissolution, has little gastrointestinal irritation, and is not prone to the formation of kidney stones. It is suitable for patients with gastric acid deficiency and risk of kidney stones. Calcium supplementation needs to be appropriate to avoid excessive calcium supplementation, which may increase the risk of kidney stones and cardiovascular diseases. Patients with hypercalcemia and hypercalciuria should avoid calcium supplementation. In the treatment of CTIBL, calcium should be used in combination with other medications [25].
-
Vitamin D: Vitamin D promotes calcium absorption, which helps maintain bone health, preserve muscle strength, improve balance, and reduce the risk of fractures. People with vitamin D deficiency or insufficiency can try taking 1000–2000 IU of vitamin D3 orally daily to maintain serum 25OHD levels above 30 µg/L [26]. The use of active vitamin D or its analogs does not correct vitamin D deficiency or insufficiency; single large oral doses of conventional vitamin D are not recommended as a supplement [18].
-
- c.
Bone-modifying agents
-
Mechanism of action: Bone-modifying agents include bisphosphonates and denosumab. Bisphosphonates inhibit osteoclast-mediated bone resorption by binding to hydroxyapatite in bone, reducing bone loss and enhancing bone density [25]. Different bisphosphonates have different improvements in bone density and bone resorption, as well as fracture prevention [23]. Denosumab is a fully humanized monoclonal antibody (immunoglobulin G2) that specifically binds to the receptor activator of NF-κB ligand (RANKL), which targets the nuclear factor-κB receptor. It inhibits the differentiation of osteoclast precursors into osteoblasts, thereby inhibiting the formation of osteoclasts, and increases bone density and bone strength by interfering with the activation of RANKL protein and blocking the interaction between RANK and RANKL [27].
-
Drugs and Usage: Bisphosphonates are available in oral and intravenous formulations. Oral preparations, including alendronate sodium and risedronate sodium, have strict requirements on diet and posture when taking them. And its compliance is low. As a result, 70% of patients discontinue treatment during the first year [28, 29]. Moreover, interruption of treatment will affect the efficacy of the drug, and the incidence of fractures is significantly higher than that of patients who insist on taking the drug [30]. Therefore, oral bisphosphonate treatment requires attention to patient compliance. Those who cannot adhere to the medication should make timely adjustments and switch to intravenous or subcutaneous medication. Intravenous preparations include zoledronic acid and ibandronic acid, with zoledronic acid being the most commonly used [31]. Denosumab is only available for subcutaneous injection.
Commonly used bone-modifying agents and their usage are shown in Table 2.
-
Duration of administration: It should be comprehensively judged based on the duration of adjuvant endocrine therapy and the absolute risk of fracture. If adjuvant endocrine therapy is planned to last for 5 years, bone-modifying drug therapy may be continued until the end of AI therapy, if possible [32, 33]. For patients receiving adjuvant endocrine therapy for more than 5 years, an individualized risk-benefit assessment of fracture risk versus potential side effects of long-term therapy is needed. If the risk of fracture is still high, the treatment time can be appropriately extended [18, 34].
-
| Drug name | Dosage |
|---|---|
| Alendronate sodium | 70 mg/tablet, 1 tablet per week, orally. Take the drug on an empty stomach in the morning. Stay in an upright position for 30 min after taking the drug. Avoid lying down. Do not take other food or drugs within 30 min. |
| Risedronate sodium | 35 mg/tablet, 1 tablet per week; 5 mg/tablet, 1 tablet each time, once a day, orally. Same as alendronate sodium. |
| Ibandronic acid | (1) Tablet: 150 mg/tablet, 1 tablet per month, orally. (2) Injection: 2 mg Intravenous injection (IV), once every 3 months. |
| Zoledronic acid | 4 mg IV every 6 months; also consider 5 mg IV once yearly. |
| Denosumab | 60 mg subcutaneously every 6 months. |
2.1.5 Considerations on the prevention and treatment of CTIBL in patients with early breast cancer
2.1.5.1 Monitoring of BMD and biochemical bone markers
BMD should be routinely tested before OFS or AI treatment. For patients requiring only calcium and vitamin D supplementation, risk factors and BMD can be assessed every 1–2 years. BMD should be evaluated annually, and the frequency of BMD monitoring may even be increased to every 6–12 months where appropriate [35]. In addition, changes in biochemical bone markers significantly preceded changes in bone density. If conditions permit, the baseline levels of bone biochemical markers can be detected before treatment and reviewed every 3–6 months during treatment to dynamically understand their changes and help determine drug efficacy and patient treatment compliance [18].
2.1.5.2 Management of patients with suppressed ovarian function
In premenopausal women with ovarian suppression due to chemotherapy or denervation, bone loss occurs after 6 months of ovarian suppression and accelerates after 12 months [11]. Osteoporosis and fractures were observed in premenopausal patients with OFS combined with either tamoxifen or AI, and there was no significant difference in incidence between the combined AI and tamoxifen groups in the TEXT-SOFT study [36]. Therefore, BMD and risk factors should be assessed in patients with ovarian suppression, and patients who meet the requirements should be treated with bone-modifying agents.
2.1.5.3 Increased rebound bone resorption after discontinuation of denosumab
Some data show that BMD decreased rapidly after denosumab was discontinued, and the incidence of vertebral fractures increased compared with the nondrug group, which may be due to increased bone resorption rebound after drug withdrawal [37]. Therefore, bisphosphonates or other drugs should be administered sequentially after discontinuation of denosumab to prevent BMD decline and increased fracture risk [20].
2.1.5.4 Selection of endocrine drugs
It is recommended to choose endocrine drugs that have the least impact on bone safety to minimize the occurrence of bone safety problems. Selective estrogen receptor modulators (SERM) have less impact on bone loss than AI, so such drugs as triamcinolone acetonide and toremifene are the drugs of choice. Steroidal AI (exemestane) has a unique androgen-like structure and has less impact on bone safety than nonsteroidal AIs (anastrozole and letrozole), with a faster increase in bone density observed after bisphosphonate treatment [38, 39]. Therefore, SERM analogs or steroidal AIs may be considered in high-risk patients requiring a switch to endocrine therapy.
2.2 Use of adjuvant bisphosphonates and other bone-modifying agents to prevent bone metastasis and improve overall survival in early breast cancer
2.2.1 Background
The overall prognosis of early breast cancer is good, with a 5-year survival rate as high as 83.2%, but the prognosis of advanced breast cancer is relatively poor, with a 5-year survival rate of only 20% [40]. Bone is the most common site of metastasis in breast cancer patients [41]. An observational study of more than 7000 patients with early-stage breast cancer found that 22% developed bone metastases after 8.4 years of follow-up [42]. The overall survival rate and 5-year survival rate of patients with early breast cancer bone metastasis are significantly reduced [43]. The use of adjuvant bisphosphonate therapy in patients with early-stage breast cancer has attracted attention because it can help prevent bone metastasis, reduce the risk of recurrence, and even confer survival benefits to some extent.
The recommended clinical pathway for the use of bisphosphonates in the adjuvant treatment of early-stage breast cancer to prevent bone metastasis and improve survival benefit is shown in Figure 2.
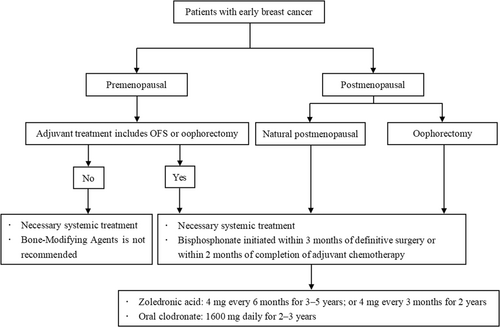
2.2.2 Adjuvant application of bisphosphonates and other bone-modifying agents in the adjuvant treatment of early breast cancer
2.2.2.1 Mechanism
Tumor cells produce cytokines and growth factors that stimulate osteoclastogenesis and induce increased bone resorption and bone matrix release of growth factors. The bone matrix releases growth factors to stimulate tumor cell proliferation, migration and angiogenesis, and bone-modifying agents can break this vicious cycle by inhibiting osteoclastogenesis and bone resorption [44]. In addition to their effects on osteoclasts and the bone microenvironment, basic research also shows that bone-modifying agents have certain antitumor activity. For example, zoledronic acid can cause intracellular accumulation of mevalonate metabolites (IPP/ApppI), activate Vγ9Vδ2 T cells, and kill tumor cells by secreting γ-interferon and perforin [45]. In addition, zoledronic acid can also exert antitumor effects by reducing tumor microvessel density [46].
2.2.2.2 Use of adjuvant bisphosphonates and other bone-modifying agents to prevent bone metastases and improve overall survival in early breast cancer
The bone metastasis prevention and survival benefits of bisphosphonates in early breast cancer focus primarily on postmenopausal and premenopausal patients treated with OFS. The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) conducted a meta-analysis of adjuvant bisphosphonate therapy for early breast cancer [47] and enrolled a total of 18,766 patients, which 9290 patients received zoledronic acid and 3072 patients received ibandronic acid. The median duration of bisphosphonate treatment was 3.4 years. It was found that almost all the benefits were restricted in patients with postmenopausal or drug-induced menopause, and adjuvant bisphosphonate therapy significantly reduced the incidence of bone metastases (p = 0.0002) and breast cancer mortality (p = 0.002) in this group of patients.
There is some controversy regarding the efficacy of denosumab in preventing bone metastases and improving survival in early breast cancer. The ABCSG-18 study showed that denosumab improved disease-free survival (DFS, p = 0.02) and bone metastasis-free survival (BMFS, p = 0.05) in hormone receptor (HR)+ early-stage breast cancer. However, there was no significant difference in overall survival (OS) between the two groups (p = 0.06) [48]. Another large phase III randomized controlled trial, the D-CARE study, showed that even with higher and more frequent denosumab doses, there was no statistical difference in BMFS (p = 0.70) and DFS (p = 0.57) between the denosumab group and the placebo control group [49].
Clinical studies of adjuvant bisphosphonates and other bone-modifying agents to prevent bone metastasis and improve overall survival in early breast cancer are shown in Table 3 [15, 49-55].
| Type | No. of patients | Administration | Duration (years) | Median followup | DFS | OS | |
|---|---|---|---|---|---|---|---|
| AZURE [50] | Multicenter, randomized, phase 3 trial | 3360 women with breast cancer (HR+/HER2+/TNBC) | ZOL+ (neo)adjuvant chemotherapy and/or endocrine therapy versus (neo)adjuvant chemotherapy and/or endocrine therapy alone | 5 | 117 months | ① ZOL in postmenopausal women improved DFS and IDFS (HRDFS = 0.82, 95% CI: 0.67–1.00; HRIDFS = 0.78, 95% CI: 0.64–0.94). ZOL in women with an MAF FISH negative tumor improved IDFS (HRIDFS = 0.75, 95% CI: 0.58–0.97), irrespective of menopause. ② Bone metastases as a first DFS recurrence (BDFS) were reduced with ZOL (HRBDFS = 0.76, 95% CI: 0.63–0.92, p = 0.005). |
ZOL in women with an MAF FISH negative tumor improved OS (HROS = 0.69, 95% CI: 0.50–0.94), irrespective of menopause. |
| ABCSG-12 [51] | Randomized, open-label, phase 3 trial | 1800 premenopausal women with HR+ breast cancer | OFS+AI/TAM+ZOL (4 mg every 6 months) versus OFS+AI/TAM | 3 | 94.4 months | The ZOL being added to endocrine therapy strongly improved DFS versus endocrine therapy alone (88.4% vs. 85.0%) for an absolute increase of 3.4%. The ZOL plus endocrine therapy was risk reduction of DFS events by 23% versus endocrine therapy alone (HR = 0.77, 95% CI: 0.60–0.99, p = 0.042) | Absolute risk reductions with ZOL were 2.2% for OS versus endocrine therapy (96.7% vs. 94.5%) |
| HOBOE [52] | Multicenter, randomized, controlled, phase 3 trial | 1065 HR+ premenopausal patients with early breast cancer | OFS+TAM versus OFS+AI versus OFS+AI+ZOL (4 mg every 6 months) | 5 | 64 months | At 5 years, the disease-free rate was respectively 85.4%, 93.2%, and 93.3% with OFS+TAM, OFS+AI, and OFS+AI+ZOL (overall p = 0.008). The hazard ratio for a DFS event was 0.52 (95% CI: 0.34–0.80, p = 0.003) with OFS+AI+ZOL versus TAM and 0.70 (95% CI: 0.44–1.12, p = 0.22) with OFS+AI+ZOL versus OFS+AI. | The overall survival rate was 96.9% (95% CI: 94.1–98.4) with OFS+TAM, 98.4% (95% CI: 96.2–99.3) with OFS+AI and 99.7% (95% CI: 97.9–100.0). |
| S0307 [53] | Randomized phase 3 trial | 6097 stage I–III breast cancer | Intravenous ZOL, oral clodronate, or oral ibandronate | 3 | 5 years | DFS did not differ across arms in a log-rank test (p = 0.49) | 5-year overall survival did not differ between arms (log-rank p = 0.50) |
| SUCCESS A [54] | Multicenter, randomized, open-label, phase 3 trial | 3754 patients with either node-positive or high-risk node-negative primary invasive BC | After completion of chemotherapy, patients were randomized again to compare 5 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years, followed by 4 mg intravenously every 6 months for 3 years) and 2 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years) | 5 versus 2 years | 35.4 months | Disease-free survival (HR = 0.97, 95% CI: 0.75–1.25; p = 0.81), and distant disease-free survival (HR = 0.87; 95% CI: 0.65–1.18; p = 0.38) did not differ significantly between the two treatment arms (5 vs. 2 years) | Overall survival (HR = 0.98; 95% CI: 0.67–1.42; p = 0.90) did not differ significantly between the two treatment arms (5 vs. 2 years) |
| BOOG 2006-04 [55] | Multicenter, randomized, open-label, phase 3 trial | 1116 postmenopausal women with stage I–III ER+ breast cancer and an indication for adjuvant endocrine therapy (ET) | ET+oral ibandronate versus ET (oral ibandronate 50 mg once daily for 3 years) | 5 years of ET and 3 years of oral ibandronate | 8.5 years | ① Adjuvant ibandronate does not improve DFS in postmenopausal patients with ER+ breast cancer (HR = 0.97,95% CI: 0.76–1.24, p = 0.811) ② Recurrence-free Interval is not significantly different between the ibandronate group and control group (HR = 0.84, 95% CI: 0.62–1.14) ③ Bone Recurrence-free Interval is not significantly different between the ibandronate group and control group (HR = 0.83, 95% CI: 0.55–1.25) |
OS was not improved by adjuvant ibandronate (50 mg/day) in postmenopausal patients with ER+ breast cancer (HR = 1.10, 95% CI: 0.82–1.49, p = 0.517) |
| ABCSG-18 [15] | Randomized, double-blind, placebo-controlled, phase 3 trial | 3425 postmenopausal patients with early, hormone receptor-positive, nonmetastatic breast cancer | denosumab (60 mg every 6 months) +AI versus placebo+AI | 3 | 96 month | ① DFS (8 years of follow-up) was improved in the denosumab group versus the placebo group (HR = 0.83, 95% CI: 0.71–0.97, p = 0.016) ② BMFS was improved in the denosumab group versus the placebo group (HR = 0.81, 95% CI: 0.65–1.00, p = 0.047) |
There is no statistical difference between two groups (HR = 0.80, 95% CI: 0.64–1.01, p = 0.065) |
| D-CARE [49] | International, multicenter, randomized, controlled, phase 3 trial | 4509 patients with stage II–III breast cancer or locally advanced disease | systemic therapy + denosumab (120 mg, once every 3–4 weeks for about 6 months and then once every 12 weeks) | 5 | 67 months | ① DFS was not significantly improved in the denosumab group versus the placebo group (HR = 1.04, 95% CI: 0.91–1.19, p = 0.57) ② BMFS was not significantly different (median not reached in either group, HR = 0.97, 95% CI: 0.82–1.14; p = 0.70). |
/ |
- Abbreviations: AIs, aromatase inhibitors; BMFS, bone metastasis-free survival; CI, confidence interval; DFS, disease-free survival; ET, endocrine therapy; HR, hazard ratio; IDFS, invasive disease-free survival; OS, overall survival; TAM, tamoxifen; ZOL, zoledronic acid.
2.2.2.3 Recommendations
- a.
Population: Adjuvant bisphosphonates therapy should be considered in all postmenopausal (natural or treatment-induced) patients with primary breast cancer, regardless of HR and human epidermal growth factor receptor 2 (HER2) status [56]. The decision to recommend adjuvant bisphosphonate therapy should be based on a comprehensive analysis of the patient's disease characteristics, recurrence risk, life expectancy, drug side effects, patient preferences and other factors, and weigh the potential benefits and risks. The NHS PREDICT tool (https://breast.predict.nhs.uk/) can assess the benefits of adjuvant bisphosphonates, such as reduction in mortality associated with adjuvant bisphosphonates, to aid treatment decisions [56, 57].
- b.
Timing: It is recommended to start bisphosphonate treatment as early as possible, that is, within 3 months after surgery or within 2 months after the end of adjuvant chemotherapy.
- c.
Drug selection and recommended dosage:
-
Zoledronic acid: 4 mg once every 6 months for 3–5 years; or 4 mg every 3 months for 2 years;
-
Oral clodronate: 1600 mg daily for 2–3 years.
- d.
Clinical application of bone-modifying agents in the adjuvant treatment of early breast cancer
For postmenopausal breast cancer patients, an expert panel at the 2023 St. Gallen International Breast Cancer Conference strongly supported the use of adjuvant bisphosphonates to improve DFS, with 80% support. However, only 42.6% of the panel experts used bisphosphonates in routine clinical treatment [58]. At the 2023 China Breast Cancer Forum, 76% of the expert group expressed “agreement” and supported the use of bone-modifying agents to improve DFS in postmenopausal patients with early breast cancer, of which 76% of the expert group preferred to choose zoledronic acid or other bisphosphonates. Although many guidelines and consensus strongly recommend the use of adjuvant bisphosphonates to improve DFS in early breast cancer [3, 20, 56, 58-60], fewer groups use bisphosphonates in clinical routine. Clinicians should make clinical treatment decisions based on weighing the pros and cons of using adjuvant bisphosphonates and fully communicating with patients.
3 BONE HEALTH MANAGEMENT FOR PATIENTS WITH BREAST CANCER BONE METASTASIS
3.1 Background
Bone is the most common and earliest metastasis site of breast cancer, especially the axial skeleton [61-63]. Fifty percent of newly diagnosed patients with advanced breast cancer have bone metastasis [53], and the incidence of bone metastasis in all patients with advanced breast cancer is as high as 75% [64]. Luminal breast cancer is more likely to develop bone metastasis than other types of breast cancer [65]. Once bone metastasis occurs, it will affect the patients' survival. Even for patients with only bone metastases, OS is still not optimistic, ranging from 26 months to 4 years [66, 67]. Once bone metastasis occurs in breast cancer patients, the most common complication is SRE, which not only reduces the patient's quality of life, but also affects their long-term survival [20]. A study that followed 35,912 breast cancer patients for 8 years found that breast cancer patients with bone metastases who developed SRE had significantly shorter survival than those who did not develop SRE [68]. Therefore, early diagnosis and treatment are of great value to the survival and quality of life of patients with bone metastases, as it can reduce or delay the occurrence of SRE.
3.2 Clinical characteristics of breast cancer bone metastasis
The clinical characteristics of breast cancer bone metastasis are single or multiple osteolytic lesions. Fifteen percent to 20% of patients develop osteoblastic lesions, and a few patients develop mixed lesions [48] SRE often occurs when bone metastases occur. SRE refers to bone pain, pathologic fractures, vertebral collapse, spinal cord compression, and hypercalcemia, as well as bone radiotherapy and bone surgery for the prevention and treatment of SRE. A Danish study showed that the incidence of SRE was highest in the first year of initial diagnosis of bone metastasis, which could be as high as 38.5% [69]. A 12-year cohort study in South Korea showed that the cumulative incidence of SRE in breast cancer patients was 47% [70].
Bone pain is the most common SRE, occurring in 80% of patients with bone metastases [65, 71]. Hypercalcemia is the most lethal SRE and is associated with poor prognosis; it occurs in 10%–30% of patients with osteolytic lesions [72]. Severe hypercalcemia can cause malaise, nausea, lethargy, muscle weakness, cardiovascular and renal dysfunction, confusion, and coma [73, 74]. The incidence of pathologic fractures is 17%–50%, and the onset time is relatively late, can cause pain, deformity, difficulty moving, paralysis, and death [74]. Ten percent of patients suffer spinal cord compression due to structural instability of the spine [75]. In severe cases, this can lead to motor and sensory dysfunction, incontinence, nerve root pain, and paralysis [76].
3.3 Diagnosis of breast cancer bone metastasis
Early diagnosis is of great significance for breast cancer staging, prevention, and treatment of SRE, and helps to minimize and mitigate the adverse effects of SRE on patients. Bone metastases need to be screened when patients have the following symptoms: (1) Bone pain or fracture, (2) symptoms of spinal cord or nerve compression, (3) elevated blood alkaline phosphatase, and (4) hypercalcemia [76]. The diagnosis of breast cancer bone metastasis requires a combination of medical history, symptoms, signs and laboratory tests, and so forth. Imaging examination is the main diagnostic method, and bone biopsy can be performed when necessary.
3.3.1 Emission computed tomography (ECT)
Bone scan is the most used method to screen for bone metastases. The advantage of ECT is that it can scan the entire skeleton and determine the location and number of bone metastases. ECT has high sensitivity, but the disadvantage is low specificity [77]. Technetium-99m (99m Tc)-methylene diphosphonate (MDP) radionuclide imaging is recommended. 99m Tc-MDP is extremely osteophilic, so it is more sensitive to osteoblastic lesions and less specific to osteolytic lesions, making it easy to be misdiagnosed and missed, leading to underreporting [78]. In addition, for some patients who are effectively treated, ECT may show increased activity or the emergence of new lesions (flare reaction) due to increased tracer absorption during the first 3 months due to the formation of new bone during the repair. After 6 months of treatment, bone scans are likely to show improvement only when the addition of immature new bone has stopped and the flare reaction has decreased. Therefore, the use of ECT to evaluate treatment response in osteolytic lesions is not recommended [20, 78].
3.3.2 Digital radiography (DR)
For patients with pain and suspected pathologic fractures, DR examination is the first choice. It can also help determine whether the tumor is an osteolytic, osteoblastic, or mixed lesion. The advantages of DR are fast and inexpensive, but the sensitivity of DR to early bone metastasis is very low. For osteolytic lesions, they need to be larger than 1 cm in diameter and have a loss of more than 50% of the bone mineral content in the lesion to be identified; individual osteoblastic lesions are more difficult to assess [20].
3.3.3 Computered tomography (CT)
CT is a better test for determining the size of bone lesions and assessing the extent of cortical bone involvement, and its sensitivity is better than radiograph. CT is not usually used as a systematic screening for bone metastases. When using CT, the size of the bone metastases must be at least 1 cm, and the bone density loss is approximately 25%–50% [71]. In addition, CT is more accurate in evaluating ribs and can also detect bone marrow metastases before bone destruction becomes apparent. Enhanced CT scans can also clearly show the blood supply of bone metastases and their relationship with adjacent blood vessels and nerves, and determine whether metastases in the spine extend into the spinal canal. CT is well suited for biopsy localization but is not suitable for whole-body scanning for bone metastasis screening [20]. Currently, CT is a commonly used method in clinical practice to evaluate treatment effects, but it cannot distinguish the metabolic status of lesions and has certain limitations.
3.3.4 Magnetic resonance imaging (MRI)
MRI has high sensitivity and specificity for detecting bone metastasis. It can accurately display the location and extent of lesion invasion as well as the invasion of surrounding soft tissues. MRI is more sensitive than bone scan for detecting spinal metastases and can be used to assess the integrity and the ultimate compression status of the spinal cord [20, 71]. In addition, MRI can detect bone marrow involvement before the occurrence of osteoblastic lesions, and its resolution is higher than that of CT, making it the first choice of tools for evaluating intramedullary infiltration of bone metastases in breast cancer [78]. MRI can also be used to evaluate treatment effects, but due to its high cost, it is not suitable for screening bone metastases [78].
3.3.5 Positron emission tomography-computed tomography (PET-CT)
PET-CT is an excellent method for detecting bone metastases, with higher detection accuracy than CT and ECT. It has high sensitivity and specificity [71, 78]. Different radioactive tracers have different advantages for the detection of bone metastasis.18F-fluorodeoxy glucose (18F-FDG) PET-CT is the most widely used radioactive tracer. It has higher sensitivity and specificity for the visualization of osteolytic bone metastasis; 18F-NaF PET-CT is the most accurate radioactive tracer and is more sensitive to osteoblastic metastases, but it is expensive and not widely used [79]. FDG-PET-CT can accurately distinguish progressive osteosclerosis and reflect tumor progression or response to treatment through quantitative assessment of FDG uptake before, during, and after treatment. It is the best way to evaluate the response to treatment of high-metabolism bone metastases [28].
3.3.6 Bone biopsy
Pathology is the gold standard for diagnosing bone metastases in breast cancer, but not all patients with bone metastases require bone biopsy. For uncomplicated bone involvement, histologic confirmation of metastatic disease can be performed, especially when the lesions are sparse or unclear on imaging [28]. The advantage of needle biopsy is to identify metastatic lesions and provide molecular classification of metastatic lesions to guide subsequent treatment.
3.3.7 Bone biochemical markers
Bone biochemical markers can reflect the speed of bone resorption and formation, indicating the degree of bone destruction and repair during bone metastasis. Among common bone biochemical markers, markers that reflect the level of osteolytic metabolism include CTX, NTX, and so forth, and markers that reflect osteoblastic metabolism include BALP, and so forth. Elevated levels of bone biochemical markers may indicate bone metastasis, but the sensitivity and specificity are low and are not recommended for the diagnosis of bone metastasis [80].
3.4 Treatment
Breast cancer bone metastasis requires a multidisciplinary collaboration model, commonly referred to as multiple disciplinary treatment (MDT). This approach is guided by experts across various disciplines, including medical oncology, radiotherapy, orthopedics, plastic surgery, and other specialized fields. The objective is to collectively devise a rational and personalized treatment plan tailored to the specific needs of each patient. The goals of treatment are to prevent or delay the occurrence of SRE, reduce pain, restore function, control tumor progression, improve quality of life, and extend the patient's survival time as much as possible. The diagnosis and treatment process of breast cancer bone metastasis is shown in Figure 3.
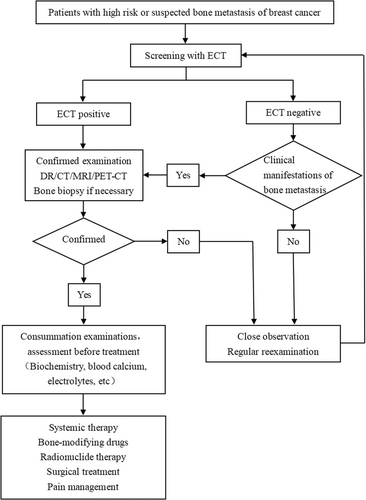
3.4.1 Efficacy evaluation
During treatment, the efficacy should be evaluated according to the treatment cycle. The evaluation of the efficacy of simple bone metastasis is mainly based on bone repair and destruction, rather than changes in tumor volume, and requires a comprehensive evaluation based on the patient's clinical manifestations and imaging examinations. The healing process of bone metastases is slow and only begins to heal after 3–6 months of treatment and takes more than a year to mature [28]. If the patient's bone pain symptoms are relieved, imaging examinations show clear boundaries and increased density of bone lesions, reduction in soft tissue mass volume, liquefaction, and necrosis in the tumor center, and ECT or PET-CT shows reduced tumor uptake, all of which may indicate that tumor treatment may be effective [81].
3.4.2 Systemic treatment
Breast cancer bone metastasis is an advanced systemic disease, and systemic therapy is the first treatment. Specific treatment strategies need to select effective antitumor treatment options based on the molecular classification of the patient's primary tumor, the characteristics of recurrent metastasis, and the molecular classification of metastases after puncture. Endocrine drugs combined with CDK4/6 inhibitors have become the standard choice for first- and second-line treatment for most patients with HR positive advanced breast cancer; However, for breast cancer with short progression-free interval, rapid disease progression, visceral crisis, or failure of multiple lines of endocrine therapy, chemotherapy is the main option. For patients with advanced HER2+ breast cancer, anti-HER2 targeted therapy is one of the important treatments. For patients with low HER2 expression, in addition to conventional treatment, antibody-drug conjugates are also a good treatment option. For triple-negative breast cancer, in addition to chemotherapy, targeted drugs, antibody-drug conjugates, and immune checkpoint inhibitors are also options.
3.4.3 Bone-modifying agents
Bone-modifying agents can reduce the incidence of SRE in patients with bone metastasis and are basic drugs for breast cancer bone metastasis. Bone-modifying agents include bisphosphonates and denosumab. It should be noted that the dose and frequency of bone-modifying agents used in the treatment of patients with advanced bone metastases are different from those used in the early prevention and treatment of osteoporosis.
3.4.3.1 Bisphosphonates
Bisphosphonates can effectively delay the occurrence of SRE (3–6 months), reduce the incidence of SRE (30%–40%), improve bone pain (50%), and are also an effective treatment for malignant hypercalcemia [82], but it cannot improve the survival rate of patients with bone metastasis [83]. Currently, there are three generations of bisphosphonate drugs.
First-generation bisphosphonate drugs: Represented by clodronate disodium, there are currently intravenous and oral preparations available. Dosage and administration: The recommended oral dosage is 1600 mg/day; Alternatively, an initial intravenous dose of 400 mg/day of clodronate disodium can be administered via intravenous infusion lasting more than 2 h for 3 consecutive days, followed by the continuation of clodronate disodium at 1600 mg/day orally, completing a cycle over a total duration of 3–4 weeks.
Second-generation bisphosphonate drugs: Represented by pamidronate disodium, the in vitro activity of the drugs in inhibiting bone resorption is stronger than that of the first-generation drugs. Dosage and administration: Pamidronate disodium 60–90 mg, intravenous infusion last more than 2 h, once every 3–4 weeks.
Third-generation bisphosphonate drugs: Mainly including zoledronic acid, ibandronic acid, and incadronic acid, the intensity and efficacy of which are further improved compared with the second generation. Dosage and administration: Zoledronic acid 4 mg, intravenous infusion lasting more than 15 min; Ibandronic acid 6 mg, intravenous infusion lasting more than 2 h; Inkadronic acid 10 mg is the general dose, and the recommended dose for patients over 65 years old is 5 mg once intravenously infusion >2 h. The above drugs are administered once every 3–4 weeks.
3.4.3.2 Denosumab
Denosumab can effectively delay the onset of SRE, reduce the risk of SRE in patients with breast cancer bone metastases by 23% [84], and delay the occurrence of moderate to severe pain [85]. Phase III randomized controlled studies and meta-studies have shown that its efficacy is superior to zoledronic acid, and the quality of life of patients treated with denosumab is also superior to those treated with zoledronic acid [71, 83, 84]. In addition, denosumab is not metabolized or excreted by the kidneys, making it a better choice for patients with renal dysfunction or combined use of nephrotoxic drugs such as platinum. Dosage and administration: 120 mg subcutaneously every 4 weeks.
3.4.3.3 Timing to start medication
For patients expected to survive ≥3 months, regardless of whether there are symptomatic, it is recommended to start medication when imaging findings of bone metastases appear [86, 87]. Bone-modifying agents can be added to systemic treatment of breast cancer bone metastases.
3.4.3.4 Adjustment of dosing intervals
It is recommended that bisphosphonates such as zoledronic acid be routinely administered every 3–4 weeks. Meta-analysis showed that the efficacy of zoledronic acid administered every 12 weeks was not inferior to that administered every 4 weeks, but more patients in the 12-week dosing group underwent bone surgery [88]. Therefore, for patients with stable disease, the consideration of zoledronic acid injection every 12 weeks may be appropriate, after 12 consecutive doses. Detumomab is administered every 4 weeks and should not be interrupted as it is not stored in the bone and interruption of treatment may be risky [20].
3.4.3.5 Dosage duration
It is recommended to use it for more than 2 years. The interval can be extended as appropriate for long-term use.
The median duration of bisphosphonate treatment of bone metastases is 9–18 months. There is currently a lack of clinical research data on medication for more than 2 years, but this should not be a contraindication for long-term medication in clinical practice [89]. Even if SRE occurs during treatment with bone-modifying drug, it is recommended to continue treatment and consider long-term use of the drug based on patient benefit [90]. Relief of bone pain does not indicate the need to discontinue. If a clear serious adverse drug event occurs or the clinician believes that the patient will not benefit from continued use of the drug, the drug should be discontinued or switched to another bone-modifying drug.
3.4.3.6 Precautions
- a.
Bone-modifying agents can be used in combination with radiotherapy, chemotherapy, endocrine therapy, and analgesics.
- b.
The recommended interval for switching to denosumab after discontinuation of bisphosphonates is 4 weeks.
- c.
If denosumab is discontinued for more than 6 months, it is recommended to give sequential treatment with bisphosphonates (e.g., zoledronic acid) to inhibit the rebound of bone resorption [20].
- d.
From a safety perspective, the combined use of bisphosphonates and denosumab is not recommended.
- e.
Inhibition of bone resorption may lead to hypocalcemia, which is most obvious when using denosumab [20].
3.4.4 Radiotherapy
3.4.4.1 External radiation therapy
Palliative radiation therapy is an effective treatment for palliation of breast cancer bone metastases, with treatment goals including pain relief, bone recalcification and stabilization, reduction of spinal cord compression and relief of neurological symptoms, and prevention of functional disability and adverse bone events [91].
In addition, preventive radiation therapy may also be used in asymptomatic individuals with weight-bearing bones to reduce the occurrence of SRE. At the same time, for long-term survival of patients with oligometastasis, local radiotherapy can further consolidate the effect of systemic therapy. Pain relief is the most common indication for radiotherapy. Radiotherapy has a high pain relief rate, and radiotherapy may be considered for patients with poor pain relief, persistent pain, or recurrent pain [20, 92, 93]. For bone calcification and bone stability, radiotherapy combined with bone improving drugs is more effective, and 80% of patients can achieve treatment goals [92]. For patients with spinal cord compression, radiotherapy may be considered. Patients with spinal cord compression should receive immediate emergency treatment, with surgical and radiation treatment plans evaluated by a surgeon and radiologist together. Patients who are not suitable for surgery can receive radiotherapy alone [20, 92]. Commonly used radiotherapy techniques include three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and stereotactic radiotherapy [94].
3.4.4.2 Radionuclide therapy
Radionuclide therapy is mainly used for pain control of systemic bone metastases, especially for the treatment of refractory bone pain, and is often used for osteoblastic lesions. Radionuclide therapy may be considered if the patient has multiple painful sites of osteoblast metastasis within an anatomical region that is larger than the area that can be safely treated with external beam radiation [95]. 89Sr is the most commonly used radionuclide drug in internal irradiation radiotherapy for bone metastases from breast cancer [96]. Some patients will experience significant bone marrow suppression and slow recovery after radionuclide therapy, which will affect subsequent systemic treatments such as chemotherapy. Radionuclide therapy should strictly control the indications and is not the first choice.
3.4.5 Surgical treatment
Surgery is a very important palliative treatment measure for patients with symptomatic bone metastases. The roles of surgery include (1) preventive fixation to prevent impending pathological fractures, (2) stabilization of pathologic fractures, (3) segmental resection of tumors, and (4) replacement of joints destroyed by tumors [97]. Surgery can help preserve or restore bone integrity and limb function, prevent or eliminate neurologic damage, and thereby improve patients' quality of life [98, 99]. Multidisciplinary evaluation including orthopedics, medical oncology, and radiotherapy is required before surgery, based on the patient's tumor characteristics, the number of metastases and their anatomical location, life expectancy, and the patient's expectations and activity level [100].
Preventive internal fixation before fracture has the best functional recovery effect [100] and is, therefore, the first choice. This requires effective judgment of the timing and method of surgery, and strives to treat the fracture before it occurs and before paraplegia, so as to avoid unnecessary pain for the patient [101]. Risk factors that may predict fracture include severe pain, lesion length greater than 2.5 cm, cortical bone destruction greater than 50%, Harrington criteria, and Mirels criteria [100, 102-104].
Bone metastasis fractures can occur in long bones, vertebrae, pelvis, and so forth, and treatment methods vary depending on the location. The main choices are intramedullary nailing, cephalomedullary nailing, semiarthroplasty, or total joint replacement with prosthesis or osteoplasty [105]. Pathologic fractures of the extremities have a significant impact on the patient's functional mobility, and surgery is recommended for impending and existing pathologic fractures of the long bones of the extremities [99, 106]. The spine and pelvis are among the most common areas affected by metastasis [100]. Treatment of pathological spinal fractures requires consideration of the extent and characteristics of the neurologic injury, the patient's overall condition, and the expected oncologic outcome. Patients with severe but incomplete neurologic deficits, recent symptoms and a favorable prognosis are most likely to benefit from surgery [106]. Surgery for pelvic metastases is challenging due to the complex bony anatomy and adjacent vital structures, requiring the surgeon to select an appropriate surgical approach based on the pelvis region and fracture type [97-107].
3.4.6 Pain management
- a.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen: They are the first choice basic drugs for pain relief treatment, and common ones include aspirin, ibuprofen, and so forth. They are often used to relieve mild pain or in combination with opioids to relieve moderate to severe pain. The FDA recommends that patients use up to 4 g of acetaminophen per day.
- b.
Opioids: They are the first choice drugs for the treatment of moderate to severe cancer pain. It is recommended to choose opioid receptor agonists. It should be taken orally for long-term use. Transdermal absorption administration and temporary subcutaneous injection can be used when there are clear indications. Patient-controlled analgesia can be given when necessary. The rescue dose is 10%–20% of the total dose taken in the previous 24 h. If rescue doses of short-acting opioids are given ≥3 times per day, consideration should be given to converting the rescue dose in the first 24 h to long-acting opioids to facilitate on-time administration.
4 DRUG SAFETY MANAGEMENT
4.1 Treatment of drug-related osteonecrosis of the jaw
4.1.1 Clinical incidence
Osteonecrosis of the jaw due to the use of bisphosphonates, denosumab, or antiangiogenic drugs such as sunitinib is collectively known as medication-related osteonecrosis of the jaw (MRONJ) [108]. The pathogenesis of MRONJ has not been fully elucidated, but it may be related to the high bone metabolism of the jaw and the special environment of the oral cavity. The use of bone-modifying agents is one of the risk factors for MRONJ, and its mechanism may be related to inhibition of osteoclast activity, localized infection or trauma in the oral cavity, and the risk of MRONJ is related to the duration of drug use [109, 110]. Studies have shown that the incidence of MRONJ is similar with bisphosphonates and denosumab, approximately 1% per year, based on monthly treatment regimens [111].
4.1.2 Clinical management
Most cases of MRONJ reported in studies of bone-modifying agents were asymptomatic or mild to moderate, with more than 50% of all cases resolving with oral irrigation or antibiotic treatment [111]. The American Association of Oral and Maxillofacial Surgeons (AAOMS) has made recommendations on the staging criteria and treatment strategies for MRONJ [112, 113]. The treatment recommendations for osteonecrosis of the jaw are shown in Table 4. If MRONJ occurs after using bone-modifying agents, it is recommended to discontinue use of these drugs and consult a maxillofacial surgeon. Early treatment can be conservative or minor surgical treatment, but during clinical diagnosis and treatment, surgical treatment, such as mandibular segmental osteotomy or partial maxillary resection, can be considered for later stage MRONJ.
| Clinical staging | Staging criteria | Treatment strategy | Posttreatment observation |
|---|---|---|---|
| Risk period | No apparent necrotic bone in patients treated with bone-modifying agents | No treatment indicated patient education | |
| 0 period | No clinical signs of necrotic bone, but nonspecific clinical findings, radiographic changes, and symptoms | Symptomatic treatment, including pain medication and antibiotics | |
| Phase 1 | Exposed and necrotic bone or fistula that probes to the bone with no sign of infection (this period can last from months to years) | Local wound care to exposure bone. Antimicrobial mouth rinses. Removal of mobile/well-formed sequenstrum. Marginal resection is feasible for disease located above neurovascular canal. Alveolectomy is feasible for diseases located inferior to the sinus floor |
Treatment may be discontinued when the disease is remission; current nonsurgical treatment may be continued when the disease is stabilized. Reassessment after disease progression and select appropriate treatment based on the stage of disease after progression |
| Phase 2 | Exposed and necrotic bone or fistula that probes to bone, with signs of infection such as pain, and erythema at the site of the lesion, with or without purulent drainage | Local wound care to exposure bone. Antimicrobial mouth rinses. Removal of mobile/well-formed sequenstrum. Systemic antibiotics. Pain Control. Segmental resection for disease located at or below neurovascular canal in an atrophic or edentulous mandible. Partial infrastructure maxillectomy for disease located at or superior to floor of maxillary sinus. |
Treatment may be discontinued when the disease is remission. Disease stabilized or downgraded to stage I, current nonsurgical treatment may be continued; Reassessment after disease progression and select appropriate treatment based on the stage of disease after progression |
| Phase 3 | Exposed and necrotic bone or a fistula that probes to bone with infection. Pain with ≥1 of the following: exposed and necrotic bone extending beyond the alveolar bone region (e.g., inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) leading to pathologic fracture, extraoral fistula, oral antral or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor. | Removal of mobile/well-formed sequenstrum. Segmental resection for mandibular disease. Partial or total maxillectomy for maxillary disease. |
Treatment may be discontinued when the disease is remission; Stabilization of the disease may allow continuation of current nonsurgical treatment; Surgery may be considered if progression |
The incidence of MRONJ can be effectively reduced through preventive measures, including patient education, dental examination before medication, preventive dental treatment, and avoidance of traumatic dental surgery during medication [114]. It is recommended that patients complete basic periodontal treatment, dental examination, extraction of teeth that cannot be retained and other oral surgeries before bone modification drug treatment, and should wait at least 4–6 weeks after tooth extraction to wait until the bone has basically healed before starting drug treatment [115, 116]. During the period of application of bone-modifying agents, patients are advised to take good care of their own oral hygiene and have regular dental follow-up visits every 6 months. Members of the multidisciplinary team should address risk factors for MRONJ early, including poor oral health, traumatic dental procedures, ill-fitting dentures, poorly controlled diabetes, and smoking [117]. If tumor-related treatment is required on the affected tooth, noninvasive dental procedures designed to remove the infected lesion (e.g., fillings, root canal therapy) are recommended. Regular noninvasive periodontal treatment is also highly recommended. If a patient requires an extraction or other invasive dental procedure, consideration should be given to suspending bone-modifying medications, using minimally invasive extraction techniques, and adequately sealing the extraction socket with soft tissue. Postoperatively, patients should be evaluated by a dental specialists every 6–8 weeks until the surgical site is completely covered with mucosa, and antibacterial mouthwash and systemic antibiotics should be used to minimize the risk of MRONJ [109, 116, 117]. Osteonecrosis of the jaw may still occur after discontinuation of bone-modifying agents, and oral status should be followed regularly after treatment is completed [116, 118].
4.2 Management of hypocalcemia
4.2.1 Clinical incidence
Hypocalcemia may occur during treatment due to the inhibitory effect of bone-modifying agents on osteoclasts. Hypocalcemia is defined as a total plasma calcium concentration <8.8 mg/dL (<2.20 mmol/L) or a plasma calcium ion concentration <4.7 mg/dL (<1.17 mmol/L) in the presence of normal plasma protein concentrations. Common clinical manifestations of hypocalcemia include paresthesia, tetany, and, in severe cases, epilepsy, encephalopathy, and heart failure. According to the literature, the incidence of hypocalcemia caused by bone-modifying agents varies widely, ranging from 1% to 39% [119]. In addition, treating hypercalcemia caused by bone metastases from solid tumors may lead to the occurrence of hypocalcemia, which has a low incidence in the Chinese population and is therefore easily overlooked by clinicians.
4.2.2 Clinical management
Although the incidence of hypocalcemia caused by bone-modifying agents in the Chinese population is lower than that in foreign populations, it still needs to be emphasized that calcium should be supplemented as early as possible during treatment, and serum calcium or calcium ion levels should be monitored regularly to avoid severe hypocalcemia. During the treatment of bone-modifying agents, especially during the initial treatment phase, electrolyte levels should be monitored and electrocardiograms should be checked regularly [120]. Studies have shown that denosumab caused a higher incidence of hypocalcemia than zoledronic acid; Therefore, serum calcium levels should be monitored closely during denosumab treatment, especially during the first few weeks of treatment [121]. Calcium and vitamin D supplementation in combination with deslumizumab significantly reduced the incidence of hypocalcemia [122]. The primary goals of hypocalcemia treatment are to correct hypocalcemia, control symptoms, and avoid serious complications. Therefore, it is recommended that patients receiving bone-modifying agents (except those who develop hypercalcemia) supplement with at least 500 mg of calcium and 400 IU of vitamin D daily and regularly monitor serum calcium or calcium ion levels [120, 123].
4.3 Management of adverse renal reactions
4.3.1 Clinical incidence
Bone-modifying agents can directly or indirectly cause varying degrees of kidney damage, mainly found in bisphosphonates. Studies have shown that the incidence of drug-related renal adverse reactions in patients with early breast cancer was 8.8% when adjuvant zoledronic acid was used, and 10.5% for ibandronic acid [53]. In the Chinese population, the incidence of drug-related renal adverse reactions in patients with advanced breast cancer was 0.7% for short-term (≤24 months) use of zoledronic acid and 1.1% for long-term use (>24 months) [124]. Denosumab is mainly metabolized through the cellular lysosomal system, and renal function has no significant impact on the pharmacokinetics and pharmacodynamics of denosumab [125].
4.3.2 Clinical management
To prevent adverse renal reactions caused by bisphosphonates, the patient's renal function and whether there are underlying kidney-related diseases should be assessed before use. Renal function should be monitored regularly during medication. Renal damage should be detected in a timely manner and necessary intervention measures should be taken as early as possible. If there are no abnormalities in multiple consecutive evaluations, the evaluation interval can be appropriately extended. Monitoring indicators include glomerular filtration rate and proteinuria, and creatinine clearance (80–120 mL/min) is used to judge renal function. Other preventive measures include appropriately extending the infusion time, avoiding concomitant use with drugs that have adverse effects on the kidneys, and adjusting drug dosage if necessary [126, 127].
In most cases, bisphosphonate-induced acute kidney injury can be reversed with effective treatment, and a small proportion of patients may convert to chronic kidney disease [128]. When acute kidney injury occurs associated with antineoplastic drugs or bone-modifying agents, drug dosage adjustments need to be made (see Table 5). For patients with mild to moderate renal insufficiency (creatinine clearance 30–80 mL/min), there is no need to adjust the dose of pamidronate, and the recommended infusion time is >4 h; For patients with severe renal insufficiency (creatinine clearance <30 mL/min), zoledronic acid or pamidronate is not recommended, and the dose of ibandronic acid needs to be reduced to 2 mg, and the infusion time should be >1 h [129]. In patients with creatinine clearance <30 mL/min, incadronic acid needs to be used with caution or the dose reduced and renal function monitored. Denosumab is not metabolized by the kidneys. When patients experience decreased renal function, other causes should be fully investigated, and there is no need to adjust the denosumab dose immediately. If the patient still experiences a decrease in creatinine clearance 2 weeks after adjusting the dosage regimen, and renal function indicators such as persistent proteinuria or 24 h urine protein quantity >1 g of have not yet recovered, it indicates that the patient has a certain degree of renal dysfunction. In case of kidney injury, clinicians should seek consultation with a nephrologist as soon as possible for early intervention.
| Name of drug | Creatinine clearance (mL/min) | Recommended drug dosage (mg) |
|---|---|---|
| Zoledronic acid | >60 | 4.0 |
| 50–60 | 3.5 | |
| 40–49 | 3.3 | |
| 30–39 | 3.0 | |
| <30 | − | |
| Pamidronic acid | >30 | 90.0 |
| <30 | − | |
| Ibandronic acid | 50–80 | 6.0 |
| 30–50 | 4.0 | |
| <30 | 2.0 | |
| Incadronic acid | >30 | 10.0 |
| <30 | 5.0 |
- Note: “−” is not recommended.
4.4 Management of influenza-like symptoms
4.4.1 Clinical incidence
Influenza-like symptoms refer to symptoms such as fatigue, malaise, muscle pain, osteoarticular pain, and elevated body temperature that occur during bone-modifying medication. They usually last no more than 72 h and mostly occur after the first treatment. The mechanism of occurrence is not clear. The incidence of influenza-like symptoms is approximately 20.2%–27.3% with zoledronic acid and approximately 8.7% with denosumab [120, 130].
4.4.2 Clinical management
The incidence of influenza-like symptoms is higher than other adverse reactions, but most of them are transient and can be significantly relieved by symptomatic treatment, and generally do not require preventive drugs. For patients with body temperature <38.5°C, physical cooling can be used; For patients with body temperature ≥38.5°C, it is recommended to give antipyretic and analgesic drugs, fluid rehydration, and other symptomatic supportive treatments; For patients with persistent fever, dexamethasone and other hormonal drugs can be considered [131].
5 SUMMARY
Breast cancer bone health management covers the entire treatment process for patients with early and advanced breast cancer. Good management of the full cycle of bone health can bring survival and quality of life benefits to patients and should be given the necessary attention. Whether it is CTIBL or bone metastasis, early prevention, early diagnosis, and early treatment are the best treatment decisions. As for the adjuvant use of bone-modifying agents to prevent breast cancer metastasis and improve prognosis, further exploration of biomarkers to predict patient benefit is needed. Moreover, in the process of bone health management, in addition to formulating appropriate treatment strategies based on the patient's specific conditions, attention should also be paid to dealing with adverse drug reactions so that treatment can proceed smoothly. Of course, there are still some issues in the clinical treatment process that cannot be covered by the guidelines due to the lack of evidence-based medicine evidence. It is necessary for clinicians to weigh the pros and cons based on the patient's condition and situation to make individualized treatment decisions.
AUTHOR CONTRIBUTIONS
Fei Ma: Project administration (Lead); writing-review and editing (Lead). Xiuqing Shi, Jiani Wang: Writing original draft (lead)—review and editing (Equal). Li Cai, Jiayi Chen, Qianjun Chen et al.: Writing—review and editing (Equal).
ACKNOWLEDGMENTS
Guidelines Consultants (in alphabetical order of last name)
Robert E. Coleman (Department of Medical Oncology, Department of Oncology and Metabolism, The University of Sheffield), Guixing Qiu (Department of Orthopedic, Chinese Academy of Medical Sciences and Peking Union Medical College), Erwei Song (Department of Breast Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University), Binghe Xu (Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College).
Expert committee (in alphabetical order of last name)
Li Cai (Department of Medical Oncology, Harbin Medical University Cancer Hospital), Jiayi Chen (Department of Radiotherapy, Ruijin Hospital, Shanghai Jiaotong University School of Medicine), Qianjun Chen (Department of Breast Oncology, Guangdong Provincial Hospital of Traditional Chinese Medicine), Rong Chen (Department of Gynecologic Endocrinology, Chinese Academy of Medical Sciences and Peking Union Medical College), Jiuwei Cui (Department of Breast Oncology, The First Hospital of Jilin University), Yue Ding (Department of Orthopedic, Sun Yat-sen Memorial Hospital, Sun Yat-sen University), Caiwen Du (Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen Center), Zhaoqing Fan (Department of Breast Surgery, Beijing Cancer Hospital), Jifeng Feng (Department of Oncology, Jiangsu Cancer Hospital), Peifen Fu (Department of Breast Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine), Hong Ge (Department of Radiotherapy, Henan Cancer Hospital), Wei He (Department of Endocrinology, Jiangsu Province Hospital), Xichun Hu (Department of Medical Oncology, Fudan University Shanghai Cancer Center), Yan Jiang (Department of Endocrinology, Chinese Academy of Medical Sciences, Peking Union Medical College Hospital), Feng Jin (Department of Breast Surgery, The First Hospital of China Medical University), Guohui Li (Department of Pharmacy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Jianyi Li (Department of Breast Surgery, Liaoning Cancer Hospital), Jing Li (Department of Diagnostic Imaging, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Junjie Li (Department of Breast Surgery, Fudan University Shanghai Cancer Center), Xianming Li (Department of Radiotherapy, Shenzhen People's Hospital), Xingrui Li (Department of Breast and Thyroid Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology), Yi Li (Breast Center, Shunyi Women's & Children's Hospital of Beijing Children's Hospital), Hongyuan Li (Department of Breast Surgery, The First Affiliated Hospital of Chongqing Medical University), Ning Liao (Department of Breast Surgery, Guangdong Provincial People's Hospital), Ying Lin (Department of Breast Surgery, The First Affiliated Hospital, Sun Yat-sen University), Caigang Liu (Department of Breast Surgery, Shengjing Hospital of China Medical University), Hong Liu (Department of Breast Surgery, Tianjin Medical University Cancer Hospital), Jian Liu (Department of Breast Medicine, Fujian Cancer Hospital), Yunjiang Liu (Department of Breast Surgery, The Fourth Hospital of Hebei Medical University), Zhenzhen Liu (Department of Breast, Henan Cancer Hospital), Wenping Lu (Department of Oncology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences), Jinsong Lu (Department of Breast Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine), Ting Luo (Department of Oncology, West China Hospital of Sichuan University), Binlin Ma (Department of Breast and Thyroid Surgery, The Affiliated Cancer Hospital of the Third Clinical Medical College of Xinjiang Medical University), Yue Ming (PET-CT Center, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Qinguo Mo (Guangxi Medical University Cancer Hospital & Guangxi Cancer Institute, Guangxi Cancer Hospital & Medical University Oncology School & Cancer Center), Qing Ni (Department of Breast Surgery, Guizhou Provincial People's Hospital), Quchang Ouyang (Department of Breast Medicine, Hunan Cancer Hospital), Jian Pan (Department of Stomatology, West China Hospital of Stomatology Sichuan University), Yueyin Pan (Department of Tumor Chemotherapy, Anhui Provincial Hospital), Liqiang Qi (Department of Breast Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shanxi Cancer Hospital), Guangdong Qiao (Department of Breast Surgery, Yantai Yuhuangding Hospital), Xiangyan Ruan (Department of Endocrinology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University), Yehui Shi (Department of Medical Oncology, Tianjin Medical University Cancer Institute & Hospital), Zhangjun Song (Department of Breast Surgery, Shaanxi Provincial People's Hospital), Tao Sun (Department of Oncology, Liaoning Cancer Hospital), Xiaonan Sun (Department of Radiotherapy, Sir Run Run Shaw Hospital, Affiliated Hospital of Medical School of Zhejiang University), Lichen Tang (Department of Breast Surgery, Fudan University Shanghai Cancer Center), Yuee Teng (Department of Medical Oncology, The First Hospital of China Medical University), Zhongsheng Tong (Department of Medical Oncology, Tianjin Medical University Cancer Institute & Hospital), Donggui Wan (Department of Medical oncology of Integrated Traditional Chinese and Western Medicine, China-Japan Friendship Hospital), Chuan Wang (Department of Breast Surgery, Fujian Medical University Union Hospital), Haibo Wang (Department of Breast Surgery, The Affiliated Hospital of Qingdao University), Jiayu Wang (Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Jiani Wang (Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Hao Wang (Department of Breast Surgery, Sichuan Cancer Hospital & Institute), Jing Wang (Department of Breast Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Ouchen Wang (Department of Breast Surgery, The First Affiliated Hospital of Wenzhou Medical University), Shu Wang (Department of Breast Surgery, Peking University People's Hospital), Shulian Wang (Department of Radiotherapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Shusen Wang (Department of Medical Oncology, Sun Yat-sen University Cancer Center), Ting Wang (Department of Thyroid, Breast and Vascular Surgery, Xijing Hospital, Airforce Medical University), Xiaojia Wang (Department of Breast Oncology, Zhejiang Cancer Hospital), Yongsheng Wang (Department of Breast Surgery, Shandong Cancer Hospital), Yu Wang (Department of Radiotherapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shanxi Cancer Hospital), Zhengzhen Wang (School of Sports Medicine and Sports Rehabilitation, Beijing Sport University), Jiong Wu (Department of Breast Surgery, Fudan University Shanghai Cancer Center), Nan Wu (Department of Orthopedic, Chinese Academy of Medical Sciences, Peking Union Medical College Hospital), Wenjie Wu (Department of Maxillofacial Surgery, Peking University School and Hospital of Stomatology), Xinhong Wu (Department of Breast Surgery, Hubei Cancer Hospital), Weibo Xia (Department of Endocrinology, Chinese Academy of Medical Sciences, Peking Union Medical College Hospital), Huihua Xiong (Department of Medical Oncology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology), Guiying Xu (Department of Breast Surgery, Jilin Cancer Hospital), Jin Yang (Department of Medical Oncology, The First Affiliated Hospital of Xi'an Jiaotong University), Qifeng Yang (Department of Breast Surgery, Qilu Hospital of Shandong University), Herui Yao (Department of Breast Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University), Jingming Ye (Department of Thyroid and Breast Surgery, Peking University First Hospital), Songqing Ye (Department of Breast Surgery, Fujian Provincial Hospital), Yongmei Yin (Department of Medical Oncology, Jiangsu Province Hospital), Hongyan Ying (Department of Medical Oncology, Chinese Academy of Medical Sciences, Peking Union Medical College Hospital), Hua Yue (Department of Osteoporosis and Osteopathy, Shanghai Sixth People's Hospital), Xiaohua Zeng (Department of Breast Cancer center, Chongqing Cancer Hospital), Dianlong Zhang (Department of Breast and Thyroid Surgery, Affiliated Zhongshan Hospital of Dalian University), Gangling Zhang (Department of Breast Surgery, Baotou Cancer Hospital), Jie Zhang (Department of Maxillofacial Surgery, Peking University School and Hospital of Stomatology), Pin Zhang (Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Qingyuan Zhang (Department of Medical Oncology, Harbin Medical University Cancer Hospital), Yanxia Zhao (Department of Medical Oncology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology).
CONFLICT OF INTEREST STATEMENT
Professor Fei Ma is a member of the Cancer Innovation Editorial Board. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




