The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential
Abstract
Oral squamous cell carcinoma (OSCC), the eighth most prevalent cancer in the world, arises from the interaction of multiple factors including tobacco, alcohol consumption, and betel quid. Chemotherapeutic agents such as cisplatin, 5-fluorouracil, and paclitaxel have now become the first-line options for OSCC patients. Nevertheless, most OSCC patients eventually acquire drug resistance, leading to poor prognosis. With the discovery and identification of non-coding RNAs (ncRNAs), the functions of dysregulated ncRNAs in OSCC development and drug resistance are gradually being widely recognized. The mechanisms of drug resistance of OSCC are intricate and involve drug efflux, epithelial-mesenchymal transition, DNA damage repair, and autophagy. At present, strategies to explore the reversal of drug resistance of OSCC need to be urgently developed. Nano-delivery and self-cellular drug delivery platforms are considered as effective strategies to overcome drug resistance due to their tumor targeting, controlled release, and consistent pharmacokinetic profiles. In particular, the combined application of new technologies (including CRISPR systems) opened up new horizons for the treatment of drug resistance of OSCC. Hence, this review explored emerging regulatory functions of ncRNAs in drug resistance of OSCC, elucidated multiple ncRNA-meditated mechanisms of drug resistance of OSCC, and discussed the potential value of drug delivery platforms using nanoparticles and self-cells as carriers in drug resistance of OSCC.
Abbreviations
-
- 5-FU
-
- 5-fluorouracil
-
- ABC
-
- adenosine triphosphate binding cassette
-
- AKR
-
- Aldo-keto reductase
-
- AML
-
- acute myeloid leukemia
-
- ATG
-
- autophagy-related genes
-
- BARD1
-
- BRCA1-associated RING domain protein 1
-
- BCRP
-
- breast cancer resistance proteins
-
- BER
-
- base excision repair
-
- BMSCC
-
- buccal mucosal SCC
-
- CCAT1
-
- colon cancer-associated transcript-1
-
- CDDP
-
- Cisplatin
-
- circRNAs
-
- circular RNAs
-
- DDSR1
-
- DNA damage-sensitive RNA1
-
- Dox
-
- doxorubicin
-
- DSBs
-
- DNA double strand breaks
-
- ECA
-
- ethacrynic acid
-
- EGFR
-
- epidermal growth factor receptor
-
- EHF
-
- ETS homologous factor
-
- EMT
-
- epithelial mesenchymal transition
-
- EMT-TFs
-
- EMT-activating transcription factors
-
- ERCC1
-
- Excision repair cross-complimentary group 1
-
- EZH2
-
- enhancer of zeste homolog 2
-
- FEN1
-
- Flap endonuclease 1
-
- GSH
-
- glutathione
-
- GST
-
- glutathione-S-transferase
-
- HER-2
-
- human epidermal growth factor receptor 2
-
- HNSCC
-
- Head and neck squamous cell carcinoma
-
- HOTAIR
-
- HOX transcript antisense RNA
-
- HPV
-
- Human papillomavirus
-
- HR
-
- homologous recombination
-
- HULC
-
- highly upregulated in liver cancer
-
- lncRNAs
-
- long noncoding RNAs
-
- LSCC
-
- lip SCC
-
- MALAT1
-
- metastasis-associated lung adenocarcinoma transcript 1
-
- MAR
-
- matrix attachment regions
-
- MDR
-
- multidrug resistance
-
- MEG3
-
- maternally expressed gene 3
-
- miRNAs
-
- microRNAs
-
- MMPs
-
- matrix metalloproteinases
-
- MMR
-
- mismatch repair
-
- MRP1
-
- MDR-associated protein family 1
-
- ncRNAs
-
- noncoding RNAs
-
- NER
-
- nucleotide excision repair
-
- NHEJ
-
- non-homologous end joining
-
- OCSCs
-
- Oral cancer stem cells
-
- OPMD
-
- oral potentially malignant disorders
-
- OSCC
-
- Oral squamous cell carcinoma
-
- P-gp
-
- p-glycoprotein
-
- PLAC2
-
- placenta‑specific protein 2
-
- PTX
-
- paclitaxel
-
- RA
-
- Rumenic acid
-
- RC3H2
-
- ring finger and CCCH-type domains 2
-
- SATB2
-
- special AT-rich DNA binding protein 2
-
- SATB2-AS1
-
- antisense transcript of SATB2
-
- ssDNA
-
- single-strand DNA
-
- TINCR
-
- terminal differentiation-induced noncoding RNA
-
- TLS
-
- translesion synthesis
-
- TMZ
-
- Temozolomide
-
- TRIP13
-
- thyroid hormone receptor interacting protein 13
-
- TSCC
-
- tongue SCC
-
- UCA1
-
- urothelial cancer-associated 1
-
- WISP1
-
- Wnt1-inducible signaling pathway protein 1
-
- XRCC4
-
- X-Ray Cross Complementing 4
-
- ZBTB7A
-
- zinc finger and BTB domain containing 7A protein
-
- ZNF750
-
- zinc finger protein 750
1 INTRODUCTION
Oral squamous cell carcinoma (OSCC), accounting for about 40% of head and neck squamous cell carcinoma (HNSCC), is a heterogeneous neoplasm arising from the mucosal lining of the oral cavity, including the tongue, upper and lower gingiva, oral floor, palate, and buccal mucosa [1, 2]. Due to the low rate of early diagnosis, most patients are already at advanced stages by the time of diagnosis. At present, the treatment modalities for advanced OSCC mainly include surgery, chemotherapy, radiotherapy, or combinations of these modalities. Unfortunately, despite the application of various treatment modalities in the past few decades, the five-year overall survival rate of OSCC remains at 50% [3]. Chemotherapeutic agents, including platinum drugs, 5-fluorouracil (5-FU), paclitaxel (PTX), and doxorubicin (Dox), are the most common treatment options for OSCC. However, most patients would develop drug resistance. Currently, multidrug resistance (MDR) is one of the major hurdles of failed cancer chemotherapy and contributes to poor prognosis of patients [4]. The detailed mechanisms of MDR remain to be fully elucidated.
With the development of sequencing technology, approximately 98% of the human genome is transcribed into RNA without protein-coding potential and hence is termed non-coding RNA (ncRNA) [5]. The nucleotide sequences transcribed from DNA constitute the primary structure of ncRNAs which regulate the transcriptional translation of target genes either directly or indirectly by binding to them through base-complementary pairing [6]. Furthermore, ncRNAs perform biological functions by folding to form more stable secondary or tertiary structures. For example, lncRNA maternally expressed gene 3 (MEG3), a human lncRNA, exerts its cancer suppressive effect by stimulating the p53 signaling pathway [7]. Uroda et al. [8] found that in an evolutionarily conserved region of MEG3, two distal motifs interacted to form alternative, mutually exclusive pseudoknot structures (“kissing loops”) through base complementarity. The destruction of these interactions impeded the MEG3 folding, disrupted the MEG3-dependent p53 signaling, and ultimately inhibited its cancer suppressive effect. Therefore, the anticancer effect of lncRNA MEG3 can be maintained by stabilizing “kissing loops”. According to the transcript size, ncRNAs can be roughly classified into small ncRNAs (18∼200 nt; sncRNAs) and long ncRNAs (>200 nt; lncRNAs). There are different kinds of sncRNAs, including microRNAs (miRNAs) and small nucleolar RNAs [9]. It has been confirmed that ncRNAs exert their roles in gene regulation, mRNA maturation, and protein synthesis. Moreover, ncRNA-related signaling pathways can drive specific cell biological responses by recognizing extensive molecular targets [10]. For example, zinc finger protein 750 (ZNF750), a tumor repressor, was important for improving the prognosis of SCC patients [11]. Meanwhile, lncRNA terminal differentiation-induced noncoding RNA (TINCR) could function as its downstream target for cancer suppression. Further results revealed that ZNF750 upregulated the expression level of lncRNA TINCR and suppressed the malignant phenotype of SCC [12]. In addition, ChIRP analysis results revealed that lncRNA colon cancer-associated transcript-1 (CCAT1) exacerbated the in vitro and in vivo SCC malignant phenotype by forming a complex with master transcription factors (TFs, including TP63 and SOX2) and activating the TP63/SOX2-CCAT1-EGFR cascade [13]. All of the above results suggested that ncRNAs can act as upstream or downstream regulators to drive SCC progression. Emerging evidence suggests that ncRNAs play roles not only in the development but also in the treatment of cancers [14, 15]. Thus, ncRNAs serve as imperative regulators in disease progression, prognosis, and treatment. In OSCC, Shi et al. [16] tested 260 OSCC serum samples and identified two miRNAs, namely miR-626 and miR-5100, which were closely and independently associated with OSCC prognosis. Similarly, the enhancer of zeste homolog 2 (EZH2) gene has been known to be a key oncogenic driver that repressed transcription [17]. After in vitro lncRNA ring finger and CCCH-type domains 2 (RC3H2) silencing, the expression level of EZH2 was downregulated, and proliferation, migration, and invasion of OSCC cells were inhibited, whereas in vivo lncRNA RC3H2 overexpression increased the expression level of EZH2 and significantly promoted the growth and invasion of OSCC cells by sponging miR-101-3p [18]. In another study, the knockdown of lncRNA urothelial cancer-associated 1 (UCA1) significantly intensified CDDP-induced apoptosis and chemosensitivity of tongue SCC (TSCC) cells, suggesting that UCA1 silencing could be used as a new strategy to improve the sensitivity of TSCC to CDDP [19]. In parallel, this provides new insights into the functions of ncRNAs in chemotherapy of OSCC. It can be seen that multiple ncRNAs can be therapeutic targets for commonly used chemotherapeutic agents against OSCC. However, while focusing on the synergistic treatment of OSCC with ncRNAs and chemotherapeutic agents, another problem on the horizon is gradually coming to light: ncRNAs-mediated drug resistance of OSCC.
Drug resistance against OSCC poses a serious threat to the patients’ survival. Thus, strategies to mitigate the drug resistance processes are urgently needed. On one hand, nanoparticles are most commonly employed as delivery vehicles for tumor drugs and are also widely applied to counteract the drug resistance of OSCC [20]. On the other hand, engineering self-cells could evade tumor cell defenses and reverse the processes of tumor drug resistance [21]. Notably, the combined use of other tools (including CRISPR systems) also opens new windows for the treatment of tumor drug resistance. Herein, this review detailed the emerging regulatory functions of ncRNAs in drug resistance of OSCC and the mechanisms of ncRNA-related OSCC chemoresistance, thereby, providing insights to alleviate drug resistance of OSCC. Importantly, the application of nano-delivery and self-cellular drug delivery platforms may provide directions for the development of ncRNAs-based approaches to mitigate the drug resistance of OSCC.
2 OVERVIEW ON OSCC AND CHEMORESISTANCE
OSCC presents with pathological changes in the oral mucosa [1]. Depending on the site of occurrence, OSCC can be subdivided into three subtypes: buccal mucosal SCC, TSCC, and lip SCC. The probability of occurring in the tongue area is about 35.3%, followed by the floor of the mouth (22.8%) and the gingiva (12.6%) [22]. In the past ten years, the difference in prevalence rates between men and women has been narrowing, approaching a ratio of 1.1:1 [23]. However, the total number of cases has not decreased, suggesting a gradual increase in the number of female patients. Additionally, OSCC had approximately 350,000 new cases and 170,000 deaths globally in 2018, mainly in South and East Asian countries such as India, Sri Lanka, and China [24]. Owing to its high morbidity, mortality, and histological specificity, OSCC now constitutes a global public health concern imposing an enormous burden on both individuals and society.
Multiple factors have been shown to be jointly involved in OSCC progression. Tobacco was identified as a group 1 carcinogen that contributes to OSCC and currently remains one of the most dominant risk factors for OSCC. In 2017, cigarette smoking accounted for a large share in Asia, including Indonesia, China, and Mongolia [25]. Remarkably, due to the large population base, there were more OSCC patients in China than in other countries [24] and caused a heavier burden on the country. The result of a meta-analysis that included 254 studies involving more than seven countries demonstrated a relative risk of 3.43 for OSCC in smokers compared to non-smokers [26]. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), one of the major components of cigarette smoke, is well known to be a potent carcinogen. Peng et al. [27] found that NNK upregulated the expression level of miR-944 in OSCC cells. Furthermore, miR-944 elicited pro-inflammation cytokines secretion, migration, and invasion, ultimately promoting the OSCC process. Also, alcohol is established as an independent risk factor for OSCC development. The risk of OSCC in Asians is associated with alcohol abuse, with adjusted ORs varying from 4.1 to 8.8. Moreover, smoking and alcohol consumption possess synergistic effects. In individuals who overused both, the relative risk for HNSCC was ≥15 [28]. A meta-analysis showed that HNSCC had a meta-relative risk of about 7.74 for betel quid containing tobacco and 2.56 for betel quid without tobacco in the Indian subcontinent (including India and Sri Lanka) [29]. Betel quid is a blend of areca nut, slaked lime, and betel leaf, which can be combined with tobacco, sweeteners, and/or spices. Particularly in India, betel quid serves as a predominant carrier of smokeless tobacco [30]. Human papillomavirus (HPV) is also a risk factor for the OSCC process. Notably, HPV-16 is one of the most common subtypes of HPV causing OSCC. Approximately 14.9% of OSCC patients have DNA positive for HPV-16 [31]. Therefore, the complexity of etiologies gradually becomes a major obstacle in the treatment of OSCC.
Furthermore, the pathological development of OSCC is characterized by multiple stages in the context of complex etiologies. OSCC is usually preceded by oral potentially malignant disorders (OPMD) which increases the risk of becoming or already harboring invasive carcinoma. Leukoplakia and erythroplakia are the two most common and malignant types of OPMDs [32]. Leukoplakia has no characteristic histopathology. Microscopically, leukoplakia shows hyperkeratosis, epithelial hyperplasia, and/or epithelial thickening with an annual malignant transformation rate of 2.6% [33]. Unlike leukoplakia, erythroplakia often exhibits characteristic histopathological changes, including high-grade dysplasia, carcinoma in situ, or invasive SCC. Clinically, the lesions usually appear as well-demarcated and red velvety patches. However, some lesions appear rough and granular on the surface [34]. Further result has revealed that molecular-level changes (especially in ncRNAs, including miRNAs) can identify lesions that progress to cancers. For example, combination of miR-150-5p/miR-222-3p and miR-150-5p/miR-423-5p can distinguish normal healthy individuals from leukoplakia and OSCC patients, respectively [35]. Moreover, nuclear transport was established as a key program for regulating the progression of SCC. ΔNp63α, an oncogenic transcription factor, could maintain the undifferentiated state of SCC cells by controlling their nuclear transport. Meanwhile, karyopherin-β1 (KPNB1), a nuclear transport receptor, was shown to assist ΔNp63α in performing nuclear transport [36]. Hazawa et al. [37] demonstrated that importazole (an inhibitor of KPNB1) increased the expression level of p53-upregulated modulator of apoptosis (PUMA) and decreased the expression level of nucleoporin 62 (NUP62) by attenuating ΔNp63α nuclear import, which ultimately enhanced apoptosis in SCC cells. However, it was not clarified whether ΔNp63α-mediated nuclear transport is involved the regulation of ncRNAs in the OSCC process. Perhaps it will be a direction to explore drug resistance of OSCC in the future. Additionally, the mechanisms of clinical action of molecular alterations in OSCC, including early diagnosis and treatment, remain unclear.
In the face of complex etiologies and clinical manifestations of OSCC, a variety of chemotherapeutic agents are used in clinical treatment. Cisplatin (CDDP), also known as cisplatinum, is a first-line and cellular non-specific chemotherapeutic drug prescribed for the treatment of solid cancers such as breast cancer [38], head and neck carcinoma [39], and testicular cancer [40]. CDDP can enter cells through multiple accesses and bind with genomic DNA or mitochondrial DNA to form DNA-platinum adducts, which then arrest DNA replication, inhibit cell mitosis, and induce apoptosis [41]. Given the positive tumor cytotoxicity of CDDP, it is also used in the treatment of OSCC patients [42]. Similar to CDDP, 5-FU mainly disrupts DNA replication and inhibits thymidylate synthase [43]. In OSCC, 5-FU induces apoptosis through intrinsic mitochondrial-mediated signaling pathways [44]. PTX is considered to be an antitumor drug that stabilizes microtubules and blocks mitosis [45]. In the treatment of OSCC, PTX reduces the expression level of vascular endothelial growth factor but has no significant inhibitory effect on tumor growth [46]. Therefore, PTX is often used as an adjunct drug to other anticancer drugs in the treatment of OSCC. For example, PTX enhanced the toxicity of cetuximab to OSCC cells and induced apoptosis of OSCC cells [47]. However, the use of chemotherapeutic agents in OSCC is limited by the development of intrinsic or acquired drug resistance in patients. For example, the mechanisms responsible for CDDP resistance are multifactorial, such as enhancement of DNA repair, elevation of cellular detoxification, inhibition of apoptosis, and regulation of ncRNAs [48]. Besides CDDP, other chemotherapeutic agents, such as PTX and 5-FU, have shown varying degrees of drug resistance. As it can be seen, MDR emergence undoubtedly poses a severe test for the treatment of OSCC patients. It was shown that ncRNAs could be involved in a variety of biological processes to promote tumorigenesis, including signaling protein interaction, modulation of translation, miRNA sponge, stabilizes protein complex [49]. Yan et al. [50] performed an integrated analysis and identified that in OSCC pathogenesis, elevated expression levels of hsa-miR-21, hsa-miR-31, and hsa-miR-338 were associated with cellular protein metabolic process, macromolecule metabolic process. Decreased expression levels of hsa-miR-125b, hsa-miR-133a, hsa-miR-133b, and hsa-miR-139 were associated with negative regulation of macromolecule biosynthetic process and gene expression. All of the above aberrantly expressed miRNAs were involved in OSCC pathogenesis. Furthermore, in a meta-analysis that included 15 studies with 1200 OSCC samples, nine upregulated miRNAs (miR-21, miR-455-5p, miiR-155-5p, miR-372, miR-373, miR-29b, miR-1246, miR-196a, and miR-181) and seven down-regulated miRNAs (miR-204, miR-101, miR-32, miR-20a, miR-16, miR-17, and miR-125b) were identified to be associated with poor prognosis of OSCC [51]. Specifically, the pooled hazard ratio values (95% confidence interval) related to different miRNA expression for overall survival and disease-free survival were 2.65 (2.07-3.39) and 1.95 (1.28-2.98), respectively [51]. These results suggest that ncRNAs not only promoted OSCC occurrence but were also involved in poor prognosis. Recently, ncRNAs have gradually been found to play regulatory roles in the drug resistance of the OSCC process. Therefore, elucidation of ncRNAs regulating the drug resistance of the OSCC process is urgently needed to mitigate or even avoid drug resistance.
3 FUNCTIONS AND MECHANISMS OF NCRNAS IN DRUG RESISTANCE OF OSCC
3.1 Functions of ncRNAs in drug resistance of OSCC
OSCC cells progress to drug-resistant process through multiple functional roles, of which ncRNAs appear to act as mediators. Despite broad similarities in the functional progression of drug resistance, there are differences in the functional patterns mediated by different types of ncRNAs. Herein, we present the functions of ncRNAs in intensifying or weakening drug resistance of OSCC, and highlight the significance of ncRNAs as therapeutic targets for alleviating OSCC resistance (Table 1).
| ncRNA | Expressions | Sample(s) | Targets and signaling pathways | Clinical Responses | Drugs | Reference |
|---|---|---|---|---|---|---|
| miR-214 | + | Tca8113 and Tca/CDDP cells | NM | Promotes survival | CDDP | [52] |
| miR-23a | + | Tca8113 and Tca/CDDP cells | TOP2B | Promotes survival | CDDP | [52] |
| miR-21 | - | Tca8113 and Tca/CDDP cells | NM | Promotes survival | CDDP | [52] |
| miR-372 | + | SAS, OC-3, OECM-1, HSC-3 and FaDu cells | miR-372/ZBTB7A/TRAIL-R2 axis | Anti-apoptosis | CDDP; taxol | [53] |
| miR-21 | + | HSC-3-R and SCC-9-R cells | PTEN and PDCD4 | Promotes metastasis | CDDP | [54] |
| miR-365-3p | - | OC-3, CGHNC-9, and C9-IV3 cells; clinical tumor tissues | miR-365-3p/EHF/KRT16/ β5-integrin/c-Met signaling pathway | Promotes metastasis and stemness | 5-FU | [55] |
| miR-221 | + | SCC-4 and SCC-9 OSCC cells | miR‑221/TIMP3 axis | Anti-apoptosis | Dox | [56] |
| miR-371 | + | SAS cells | AKT, β-catenin, and Src | Anti-apoptosis | CDDP; taxol | [57] |
| miR-373 | + | SAS cells | AKT, β-catenin, and Src | Anti-apoptosis | CDDP; taxol | [57] |
| miR-654-5p | + | Tca-8113 and CAL-27 cells; primary fresh OSCC tissues | miR-654-5p/GRAP/Ras/Erk signaling pathway | Promotes proliferation | CDDP; 5-Fu | [58] |
| miR-1246 | + | SAS, GNM, OC-3 and Fadu cells | miR-1246-/CCNG2 axis | Enhances stemness | CDDP | [59] |
| lncRNA HOXA11-AS | + | TSCCA, CAL-27, SCC-9 and Tca8113 cells; OSCC tumor tissues | HOXA11-AS/miR-214-3p/PIM1 axis | Promotes proliferation | CDDP | [62] |
| lncRNA UCA1 | + | Tca8113, TSCCA, CAL-27 and SCC-9 cells; OSCC tumor tissues | UCA1/miR-184/SF1 axis | Promotes proliferation | CDDP | [64] |
| lncRNA ANRIL | + | OSCC-3, SCC-4, HSC-3 and CAL-27 cells; OSCC tumor tissues | MK/ANRIL/MRP1 and ABCC2/ caspase-3/BCL-2 axis |
Promotes proliferation; Anti-apoptosis |
CDDP | [65] |
| lncRNA HOTAIR | + | KB and CAL-27 cells | Autophagy and mTOR signaling pathway | Anti-apoptosis | CDDP |
[66], [67] |
| circITCH | - | SCC-6, SCC-9, SCC-25, HN-4 and HN-6 cells; OSCC tumor tissues | miR-421/PDCD4 Axis |
Promotes proliferation; Anti-apoptosis |
Bortezomib | [68] [69] |
- Upregulation, +; Downregulation, -; Cyclin G2, CCNG2; DNA topoisomerase II beta, TOP2B; Doxorubicin, Dox; ETS homologous factor, EHF; Grb-2-related adaptor protein, GRAP; Not mentioned, NM; Phosphatase and tensin homolog, PTEN; Programmed cell death 4, PDCD4; Proto-Oncogene serine/threonine-protein kinase, PIM1; Tissue inhibitor of metalloproteinase‑3, TIMP3; Zinc finger and BTB domain-containing 7A protein, ZBTB7A.
3.1.1 miRNAs and drug resistance of OSCC
A variety of anticancer drugs have been prescribed to treat OSCC. Platinum drugs are widely used in clinic because of their unique anticancer mechanisms and broad spectrums of anticancer activity. By using miRNA microarrays, Yu et al. [52] showed that expression levels of miR-214 and miR-23a were upregulated and accompanied by CDDP resistance, while the expression level of miR-21 was decreased with CDDP sensitivity in TSCC CDDP-resistant subline (Tca/CDDP) cells compared to CDDP-sensitive TSCC cells. miR-372 overexpression was detected in OSCC. Further, miR-372 was found to inhibit zinc finger and BTB domain-containing 7A protein (ZBTB7A), while ZBTB7A silencing increased the oncogenic potential and drug resistance of OSCC cells. Therefore, the sensitivity of OSCC to CDDP could be improved by miR-372 silencing [53]. Besides the aforementioned signaling pathways, exosomes exert synergistic roles in miRNA-mediated drug resistance of OSCC. For example, exosomes released from CDDP-resistant OSCC cells delivered miR-21 and ultimately enhanced CDDP resistance of OSCC cells [54].
For the other two chemotherapeutic agents (5-FU and Dox), miRNAs are also found to modulate the drug resistance of OSCC. Huang et al. [55] indicated that miR-365-3p enhanced OSCC chemosensitivity to 5-FU. The expression level of miR-221 was upregulated in Dox-treated OSCC cells compared to untreated OSCC cells. However, knockdown of miR-221 enhanced the sensitivity of OSCC cells to Dox [56]. Collectively, it can be concluded that miRNAs are involved in MDR regulation. Therefore, it is reasonable to target and regulate the expression levels of miRNAs to alleviate the drug-resistant process of OSCC.
3.1.2 lncRNAs and drug resistance of OSCC
A number of lncRNAs have been identified to be abnormally expressed in OSCC cells and involved in chemoresistance via regulating different target genes and biologic processes. For example, the interactions of lncRNAs mediating drug resistance of OSCC were based on the ceRNA theory that lncRNAs could act as miRNA sponges to weaken regulations of miRNAs on mRNAs [60]. Specifically, lncRNA HOXA11-AS overexpression was detected in OSCC tissues and cells compared to adjacent normal tissues and human oral keratinocytes. Mechanistically, lncRNA HOXA11-AS sponged miR-98–5p, which in turn suppress OSCC proliferation [61]. Meanwhile, lncRNA HOXA11-AS could also inhibit the expression level of miR-214-3p, and promote drug resistance of OSCC [62]. Notably, lncRNAs could also regulate exosomal miRNAs. For example, the downregulation of lncRNA X inactive specific transcript (XIST) enhanced exosomal miRNA-503 secretion and promoted cancer metastasis [63]. However, this regulatory mechanism has not been identified in OSCC and could be further explored in the future. Several oncogenic lncRNAs, such as lncRNA UCA1, lncRNA ANRIL, as well as lncRNA HOX transcript antisense RNA (HOTAIR), have been shown to participate in the drug resistance of OSCC.
LncRNA UCA1 was demonstrated to be upregulated in CDDP-resistant OSCC cells compared to CDDP-sensitive cells. Mechanistically, the expression level of miR-184 was inhibited by lncRNA UCA1. Moreover, in CDDP-resistant OSCC cells, miR-184 overexpression facilitated tumor suppression and chemosensitivity [64]. In addition to mediating the involvement of miRNAs in drug resistance of OSCC, lncRNAs may also recognize drug transporter proteins. For example, compared to normal and tumor adjacent tissues, lncRNA ANRIL was overexpressed in OSCC tissues. Meanwhile, lncRNA ANRIL silencing inhibited tumor cell proliferation, induced apoptosis, and increased CDDP cytotoxicity by impairing drug transporters MRP1 and ABCC2 [65]. Autophagy has also been shown to be involved in lncRNA-mediated CDDP resistance. For example, lncRNA HOTAIR was reported as an oncogene, which was overexpressed in OSCC cells compared to corresponding normal oral mucosa tissues and human oral keratinocytes [66]. Notably, lncRNA HOTAIR silencing inhibited cellular autophagy by downregulating expression levels of autophagy-related genes (ATG3 and ATG7), ultimately enhancing sensitivity to CDDP [67]. All of the above evidence suggest that targeted inhibition of several lncRNAs may be a potential therapeutic strategy to improve CDDP resistance in OSCC patients.
3.1.3 circRNAs and drug resistance of OSCC
It has been suggested that circRNAs are also involved in regulating the progression of drug resistance in cancers. Upregulated circITCH enhanced the toxicity of chemotherapeutic agents against drug-resistant multiple myeloma cells [68]. Similarly, the expression level of circITCH was reduced in OSCC tissues and cell lines compared to adjacent normal tissues and human oral keratinocytes, and circITCH overexpression significantly inhibited OSCC cell proliferation and induced apoptosis [69]. Therefore, it can be speculated that circRNA can also mediate the toxicity of chemotherapeutic drugs on OSCC cells. However, the mechanisms of circRNA-meditated drug resistance of OSCC need to be further explored.
Drug resistance of OSCC cells can be seen to involve a wide range of biological signaling pathways. Fortunately, mitigation of OSCC resistance by modulating ncRNAs is a reliable option. Presumably, signaling pathways identified do not yet address all barriers for drug resistance of OSCC. Therefore, more extensive and comprehensive mechanisms of ncRNAs mediating drug resistance of OSCC are expected to be elucidated.
3.2 ncRNA-mediated mechanisms of drug resistance of OSCC
Cancers with MDR exhibit several distinctive features, including elevated activity of drug-efflux transporters, high level of apoptotic threshold, enhanced DNA repair, and autophagy-induced drug degradation, which render tumor cells refractory to chemotherapy. Furthermore, the effectiveness of chemotherapeutic agents is constrained by intrinsic or acquired resistance. Differences at the genetic level are also shown in drug resistance of OSCC cells. Five hub genes (NOTCH1, JUN, CTNNB1, CEBPA, and ETS1) were identified by bioinformatic analysis. For example, a high mRNA expression level of NOTCH1 was associated with the EMT phenotype and drug resistance progression. Conversely, the knockdown of NOTCH1 reversed EMT phenotype and drug resistance progression [70]. Additionally, multiple hub genes can be independently regulated by hsa-miR-200c-3p, hsa-miR-200b-3p, hsa-miR-429, and hsa-miR-139-5p in miRNA-mRNA targeting regulatory network [71]. These results suggested that miRNAs can regulate drug resistance-associated hub genes and accelerate or weaken the OSCC drug resistance process. Thus, ncRNAs are destined to be involved in cancer drug-resistant progression. Currently, according to stages of chemotherapeutic drug action, it has been proven that ncRNAs engage in cancer drug resistance across multiple steps, including transmembrane transport proteins (activated drug efflux), epithelial-mesenchymal transition (elevated apoptosis threshold), DNA damage repair (prolonged cell survival), autophagy (enhanced drug degradation) and so on [72]. Notably, ncRNAs are involved in the drug resistance process of OSCC.
3.2.1 Transmembrane transport proteins
One of the root causes of drug resistance can be attributed to reduced drug concentrations, which are often caused by enhanced expression of drug efflux pump genes. It subsequently generates decreased drug influx, increased efflux, and drug sequestration in intracellular vesicles and compartments [73]. Furthermore, ncRNAs are also involved in the drug efflux process (Table 2). These changes mainly involve adenosine triphosphate (ATP) binding cassette (ABC) family proteins, which has been divided into seven subfamilies (ABCA-ABCG) [74]. It has been well documented that members of the ABC transporter protein family were associated with the MDR of OSCC include p-glycoprotein (P-gp/MDR1/ABCB1), the MDR-associated protein family (MRP1/ABCC1), and breast cancer resistance proteins (BCRP/ABCG2) [75]. These ABC family proteins have similar transmembrane domains (TMD) that can pump drugs out of cancer cells to reduce the concentration of drugs in cancer cells (Figure 1).
| Drug transport protein | Gene | ncRNA | Expression | Mechanisms | Clinical Responses | Reference |
|---|---|---|---|---|---|---|
| MDR1 | ABCB1 | circ_0109291 | + | MiR-188-3p, targeting ABCB1, could be sponged by circ_0109291, resulting in enhanced drug resistance. | Promotes proliferation | [80] |
| lncRNA MALAT1 | + | MALAT1 promoted DDP resistance via regulating P-gp, EMT, and the activation of the PI3K/AKT/m-TOR signaling pathway. | Anti-apoptosis | [81] | ||
| MRP1 | ABCC1 | lncRNA MALAT1 | + | MALAT1 decreased DDP sensitivity by upregulating MRP1 and MDR1 via STAT3 activation. | Promotes proliferation | [87] |
| lncRNA ANRIL | + | ANRIL regulated caspase-3/BCL-2 to elevate the MRP1 level. | Promotes proliferation | [65] | ||
| linc00518 | + | Linc00518 sponged miR-199a and thereby promoted MRP1 expression and induced drug resistance. | Anti-apoptosis | [91] | ||
| BCRP | ABCG2 | miR-495 | - | MiR-495 suppressed HOXC6 to inhibit EMT while promoting apoptosis of CSCs in OSCC by inhibiting the TGF-β signaling pathway. | Enhances stemness | [94] |
| miR-302 | - | MiR-302 inhibited BCRP expression by targeting the 3'- UTR of BCRP mRNA. | Anti-apoptosis | [95] | ||
| miR-1246 | + | MiR-1246 enhanced the CSCs via repression of CCNG2. | Enhances stemness | [59] |
- Upregulation, +; Downregulation, -; Cancer stem cells, CSCs; Cyclin G2, CCNG2; Epithelial-mesenchymal transition, EMT.
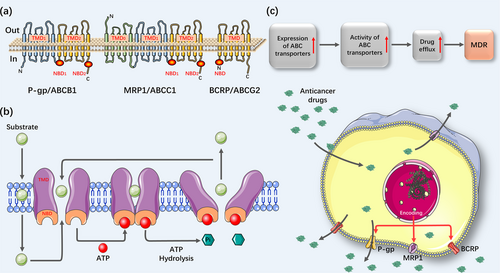
The expression level of MDR1 has been found to be upregulated in cells treated with chemotherapeutic agents. Mechanistically, anticancer drugs are capable of triggering epigenetic alterations in the promoter region of the MDR1 gene, thereby resulting in a high expression level of P-gp in tumor cells [76]. Notably, ncRNAs are responsible for the MDR1-mediated drug efflux process. For example, miR-491-3p downregulated the expression level of MDR1 by directly binding to the 3’-UTR of ABCB1, thereby enhancing the sensitivity of hepatoma carcinoma cells to drugs, like Dox or vinblastine [77]. In 1997, Jain et al. [78] first estimated immunoreactivity of P-gp in oral tissues at different stages of tumorigenesis by flow cytometry and found that its expression was significantly elevated in recurrent OSCC tissues compared to normal tissues. Meanwhile, the expression level of P-gp was higher in T4-stage compared to the T3-stage in recurrent tumors. Another result also confirmed that a high expression level of ABCB1 was correlated with high tumor grades and poor differentiation [79]. It was known that miR-188-3p, an upstream regulator of ABCB1, could be sponged by circ_0109291. Recently, Gao et al. [80] found that circ_0109291 was highly expressed in CDDP-resistant OSCC tissues and cells compared to CDDP-sensitive OSCC tissues and cells. At the same time, miR-188-3p overexpression inhibited CDDP-resistant OSCC cells. Furthermore, miR-188-3p inhibitors and ABCB1 overexpression reversed the inhibitory effect of circ_0109291 silencing on CDDP-resistant OSCC cells. Thus, circ_0109291 can increase the expression level of ABCB1 by sponging miR-188-3p and promote CDDP resistance of OSCC cells. In addition, lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) that showed high expression level in CDDP-resistant OSCC cells also promoted CDDP resistance by regulating the expression level of P-gp [81].
Since P-gp cannot explain all drug-resistant processes of cancer cells, MRP1, or ABCC1, was firstly identified in small cell lung cancer [82]. Functionally, MDR1 is mainly confined to the extrusion of xenobiotics. MRP1, on the other hand, outputs both endobiotics and xenobiotics, thereby affecting physiological processes beyond drug distribution [83, 84]. The expression level of MRP1 is known to be significantly upregulated in several drug-resistant diseases, including non-small cell lung cancer [85] and epilepsy [86]. In CDDP-resistant A549 cells, expression levels of lncRNA MALAT1 and MRP1 were upregulated compared to A549 cells. It was further found that the upregulated MRP1 was triggered by lncRNA MALAT1, thereby diminishing the sensitivity of cells to CDDP [87]. All of the above evidence points to ncRNA as a potential window in the process of alleviating drug resistance. In OSCC, the expression level of MRP1 was found to be higher in cancerous tissues than in adjacent non-neoplastic tissues. Elevated expression level of MRP1 was detected in CDDP-resistant cells compared with clinical samples, suggesting intrinsic drug resistance in OSCC. Furthermore, a high expression level of MRP1 was significantly associated with OSCC clinical stage, lymph node metastasis, and histological grade [88]. Nakamura et al. [89] also revealed that an elevated expression level of MRP1 was detected in OSCC cell lines treated with CDDP compared to untreated OSCC cells. Similar results were also observed in OSCC cells treated with vincristine [90]. LncRNA MALAT1 was also known to upregulate the expression level of MRP1, which contributed to CDDP resistance in lung cancer [87]. LncRNA MALAT1 overexpression was also detected in CDDP-resistant OSCC cells compared to CDDP-sensitive OSCC cells [81]. Therefore, it can be hypothesized that lncRNA MALAT1 can upregulate the expression level of MRP1 and diminish the drug sensitivity of OSCC cells. However, the detailed regulatory mechanisms need to be further explored.
A third drug transporter, distantly related to P-gp and MRPs, is BCRP encoded by ABCG2, which was originally isolated from drug-resistant breast cancer cell lines [92, 93]. Meanwhile, growing evidence indicates that ncRNAs can engage in BCRP-mediated drug resistance. Inhibition of BCRP expression enhanced chemosensitivity through miR-302 targeting the 3'-UTR of BCRP mRNA [95]. Yanamoto et al. [96] found that locally recurrent OSCC patients treated with neoadjuvant chemotherapy had elevated expression levels of ABCG2. Furthermore, Lu et al. [97] demonstrated that the expression level of ABCG2 was upregulated in OSCC cells with resistance to 5-FU and CDDP compared to OSCC cells. It could be concluded that the expression level of BCRP correlated with drug resistance in OSCC cells. Cells that are free of Hoechst 33342 dye are described as side population (SP) cells. This type of cells harbored cancer stem cell properties with the capacity for tumor initiation and resistance to chemotherapeutic drugs [98]. In OSCC, Zhang et al. [99] revealed the expression level of ABCG2 was higher in SP cells compared to non-SP cells. Meanwhile, it has been confirmed that a high expression level of ABCG2 in SP cells facilitated the progression of drug resistance, cancer cell proliferation, and tumor invasion in OSCC [100]. Unfortunately, intensive studies on the mechanism of ncRNA-mediated ABCG2 regulation of drug resistance of OSCC are still lacking.
3.2.2 Epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) is a reversible cellular program characterized by the loss of polarity of epithelial cells and their transformation into mesenchymal cells with the ability to move freely [101]. The EMT process is initiated by EMT-activating transcription factors (EMT-TFs), including three protein families: Snail (Snail/SNAI1 and Slug/Snai2), basic helix-loop-helix (TWIST1, TWIST2, and TCF3), and zinc-finger E-box-binding homeobox (ZEB1 and ZEB2) (Figure 2) [102, 103]. First, epithelial genes such as E-cadherin, Claudin, cytokeratin, and zona occludens 1 are suppressed whilst mesenchymal phenotype genes including Vimentin, fibronectin, N-cadherin, and matrix metalloproteinases (MMPs) are activated [104]. Then, epithelial cells lose their typical polygonal, pebbled appearance and acquire a spindle-shaped mesenchymal morphology. Subsequently, the epithelial actin architecture restructures and cells acquire motility and invasiveness by forming lamellipodia, filopodia, and invadopodia, as well as by producing MMPs to degrade extracellular matrix (ECM) proteins [105]. Further, the mesenchymal cells produced are also able to reversibly return to back to their epithelial state in a process known as mesenchymal-epithelial transformation (MET) [106]. It has been established that the EMT process plays influential roles in specific processes such as embryonic development, tissue formation, and wound healing [107]. Furthermore, activation of inappropriate EMT process contributes to malignant progression of several cancers, such as cervical cancer, OSCC, and more [108, 109].
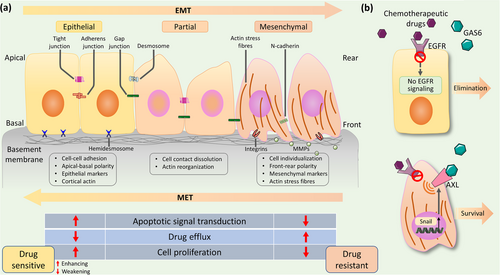
EMT is known to encompass multiple molecules and signaling pathways, and more recently ncRNAs have been shown to serve as crucial regulators of expressions and functions of EMT-TFs in OSCC pathologic processes (Table 3). For example, the expression level of circIGHG was found to be significantly upregulated in OSCC tissues compared to adjacent non-tumor tissues and was positively associated with EMT phenotype. It was further found that circIGHG induced EMT program via targeting miR-142-5p and thus, promoted OSCC progression [111]. Emerging evidence also suggested that EMT, particularly EMT-TFs, could affect the development of tumor drug resistance. EMT-TFs can bind to promoters of certain ABC transporter genes, thereby, activating the EMT program and enhancing drug resistance [112]. It can be seen that there were broad and profound mechanisms by which ncRNAs can mediate the EMT process and promote tumor drug resistance. LncRNA TINCR, a spliced lncRNA, is essential for normal epidermal differentiation. Compared with sensitive cells, the expression level of lncRNA TINCR was significantly increased in drug-resistant cells. Mechanistically, lncRNA TINCR, sponging miR-125b, stimulated human epidermal growth factor receptor 2 (HER-2) release and induced drug resistance. Furthermore, Snail-1 was a target gene of miR-125b. LncRNA TINCR silencing reversed drug resistance and EMT process by the regulation of miR-125b targeting HER-2 and Snail-1, respectively [113]. Therefore, the EMT program and tumor drug-resistant processes regulated by ncRNAs involve a variety of complex molecules and signaling pathways, with ncRNAs often acting as upstream regulators. It can be postulated that the expression levels of ncRNAs can be determinants of the EMT program and cell drug-resistance progression, and ncRNAs can serve as a potential target regulatory window in the inhibition of EMT program and tumor drug resistance.
| ncRNA | Location | Expression | Molecular mechanisms of action in EMT | Clinical Responses | Reference |
|---|---|---|---|---|---|
| circPTK2 | 8q24.3 | - | Via sponging miR-429/miR-200b-3p, circPTK2 promoted TGF-β-induced EMT and cancer cell invasion by targeting TIF1γ. | Promotes metastasis | [110] |
| circIGHG | 6q32 | + | CircIGHG directly bound miR-142-5p and consequently elevated IGF2BP3 activity. | Enhances invasion | [111] |
| lncRNA TINCR | 19p13.3 | + | LncRNA TINCR sponged miR-125b and released HER-2, and miR-125b promoted Snail-1 transcription. | Promotes metastasis | [113] |
| lncRNA SATB2-AS1 | 2q33.1 | - | After downregulation of SATB2 expression, HDAC1 failed to recruit in Snail promoter and Snail transcription was promoted. | Enhances invasion | [114] |
| miR-483-3p | 11p15.5 | - | MiR-483-3p directly targeted integrin β3, and thus repressed downstream FAK/Erk signaling pathway. | Promotes metastasis | [115] |
| miR-200b | 1p36.33 | - | MiR-200b promoted EMT through upregulation of BMI1 and enhanced tumor metastasis in chemotherapy-resistant TSCC. | Promotes metastasis | [118] |
| miR-15b | 3q25.33 | - | MiR-15b promoted EMT through upregulation of BMI1 and enhanced tumor metastasis in chemotherapy-resistant TSCC. | Promotes metastasis | [118] |
| miR-485-5p | 14q32.31 | - | Downregulation of miR-485-5p promoted PAK1 protein expression, which in turn stimulated EMT. | Enhances invasion | [120] |
- Upregulation, +; Downregulation, -; B lymphoma Mo-MLV insertion region 1 homolog, BMI1; Epithelial-mesenchymal transition, EMT; Not mentioned, NM; p21 (RAC1) activated kinase 1, PAK1.
Currently, accumulating evidence suggests complex associations between the EMT program and drug resistance of OSCC. It has been found that MDR tumor cells induced by various chemotherapeutic agents such as CDDP and epidermal growth factor receptor (EGFR) inhibitors frequently possess EMT phenotype. For example, EMT phenotype was observed in cetuximab-resistant OSCC cells with a concomitant loss of EGFR expression [116]. CDDP-resistant OSCC cell lines manifested an EMT phenotype, with decreased expression levels of E-cadherin and increased expression levels of TWIST and N-cadherin [117]. Sun et al. [118] constructed stabilized CDDP-resistant TSCC cell models and corroborated that CDDP-resistant cells exhibited mesenchymal phenotype compared to parental cells. Further results revealed that decreased expression levels of miR-200b and miR-15b contributed to chemotherapy-induced TSCC EMT phenotype. These results indicated that drug-resistant OSCC cells induced by chemotherapeutic agents tended to exhibit EMT phenotype, which predisposed residual cancer cells for being more aggressive. Notably, the EMT program also increased MDR emergence in OSCC. Snail overexpression inhibited expression levels of E-cadherin and β-catenin in OSCC cells and promoted the EMT process, as well as enhanced cellular resistance to erlotinib [119]. Lin et al. [120] found that OSCC cells with EMT phenotype induced by PAK1 (p21 (RAC1) activated kinase 1) exhibited a CDDP resistance. PAK1 took part in the invasion, migration, and cytoskeletal remodeling of OSCC cells [121]. In addition, miR-485-5p overexpression suppressed PAK1, to further reversing the EMT process. Thus, the EMT process and chemoresistance in OSCC can often coexist and mutually reinforce each other, which may involve common molecules and signaling pathways in both. Chemotherapy can induce the EMT process to promote tumor invasion and metastasis, which in turn can lead to MDR. These feedback loops collectively contribute to the malignant development of OSCC. Moreover, the paramount regulatory roles of ncRNAs in the EMT program and drug resistance of OSCC should also be considered. A better understanding of how ncRNAs regulate the EMT process and MDR can shed light on more effective ways to improve OSCC prognosis.
3.2.3 DNA damage repair
Small molecules binding to specific DNA sites are considered as potential chemotherapeutic agents. Currently, the mechanisms of many chemotherapeutic agents are to block tumor cell division by damaging DNA, such as CDDP, carboplatin, and 5-FU [122]. However, cancer cells have developed enhanced DNA repair capacity to diminish the sensitivity of cancer cells to therapy [123]. Under normal physiological conditions, multiple DNA damage responses exist for repairing damaged DNA to maintain genomic stability in cells, including base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), homologous recombination (HR), and non-homologous end joining (NHEJ) [124, 125]. In addition, cells can mobilize low-fidelity trans-lesion synthesis (TLS) DNA polymerase to bypass damaged DNA lesions and prevent cell death through the TLS system [126]. These repair mechanisms are of great value in maintaining normal cell survivals. Nevertheless, in cancer cells, these mechanisms apparently are hindrances to the efficacy of chemotherapeutic agents. Growing studies suggest that following the use of chemotherapeutic agents, enhanced DNA repair mechanisms are identified as a primary mechanism of drug resistance [127-129].
Intriguingly, alteration of molecules in signaling pathways that regulate DNA repair capacity contributes to the sensitivity and resistance of chemotherapeutic agents (Figure 3). RAD51, an important HR repair protein, can repair DNA damage caused by chemotherapeutic agents, thereby reducing the efficacy of drugs and thus, trigger MDR [130, 131]. Enhanced DNA repair capacity was observed in drug-resistant gastric cancer cells [132]. Further results showed that interferon regulatory factor-1, a tumor suppressor, could directly suppress the expression level of RAD51 via binding the RAD51 promoter, thereby impairing DNA damage repair and ultimately reversing chemoresistance [132]. The tumor suppressor complex breast cancer susceptibility gene 1; BRCA1-associated RING domain protein 1 (BRCA1-BARD1) repairs DSBs by HR signaling pathway [133]. Zhao et al. [134] indicated that the BRCA1-BARD1 complex promoted the assembly of synaptic complexes, which are key intermediates in RAD51-mediated DNA repair. It is therefore clear that BRCA1 and BARD1 are key regulators of the functions of RAD51. These results suggest that targeting molecules that regulate DNA repair signaling pathways could be an effective approach to reversing MDR in OSCC.
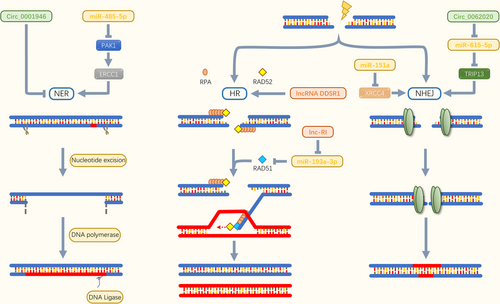
It is becoming clear that ncRNAs affect protein stability and induce drug resistance in tumor cells by regulating DNA damage-responsive genes (Table 4). As a central role in NHEJ, X-Ray Cross Complementing 4 (XRCC4) was abundant on DNA ligase 4 and bound with XRCC4-like factor to form complexes that formed alternate helical filament DNA to help cells survive [135]. Reduced expression level of miR-151a was found in temozolomide (TMZ)-resistant cells. Mechanistically, miR-151a overexpression can sensitize TMZ-resistant cells by repressing XRCC4-mediated DNA repair [137]. More recently, mounting evidence revealed that altered expression levels of lncRNAs were present in tumor cells and that lncRNA expression profiling could correlate with the evolution of tumor drug resistance [138, 139]. By using a microarray screening, Sharma et al. [140] identified a new lncRNA caused by DNA damage, termed DNA damage-sensitive RNA1 (DDSR1). LncRNA DDSR1 was further found to be involved in HR signaling pathway to enhance DNA repair. Lnc-RI silencing interfered with HR signaling pathway, increased DSBs level, and decreased the expression level of RAD51. It was further found that miR-193a-3p could bind to lnc-RI and RAD51 mRNA and block their expression. Thus, lnc-RI competitively recognized miR-193a-3p to increase the expression level of RAD51 and stabilized HR signaling pathway [141]. The expression levels of circRNAs, a type of young ncRNAs, were detected as disordered in a variety of cancers and were considered as key regulators of cancer development, invasion, and metastasis [143, 144]. In recent years, circRNAs have also been found to participate in the progression of tumor drug resistance. In prostate cancer, circ_0062020 overexpression promoted the expression level of thyroid hormone receptor-interacting protein 13 (TRIP13) via sponging miR-615-5p [146]. NHEJ signaling pathway was thus actuated, leading to enhanced DNA repair and decreased drug sensitivity of tumor cells [147]. Although the biological function of circRNAs is not fully understood, explorations of a few circRNAs have provided navigation to mechanisms of tumor drug resistance. Research on multimolecular interactions (i.e. miRNAs-lncRNAs-circRNAs) could help to fully elucidate the drug resistance mechanisms of OSCC.
| ncRNA | Expression | ncRNA targets | Mechanisms of DNA damage repair | Reference |
|---|---|---|---|---|
| miR-140 | - | FEN1 | Enhancing FEN1-mediated DNA repair | [136] |
| miR-151a | - | XRCC4 | Activating XRCC4-mediated NHEJ signaling pathway | [137] |
| lncRNA DDSR1 | + | BRCA1 | Sequestering BRCA1-RAP80 complex via direct interactions with BRCA1; Activating HR signaling pathway | [140] |
| lncRNA lnc-RI | + | RAD51; miR-193a-3p | Activating HR signaling pathway via sponging miR-193a-3p | [141] |
| lncRNA MEG3 | + | p53 | Triggering HR signaling pathway via elevating p53 levels | [142] |
| circ_0001946 | - | miR-7-5p, miR-671-5p, miR1270 and miR-3156-5p | Activating NER signaling pathway | [145] |
| circ_0062020 | + | TRIP13; miR-615-5p | Activating NHEJ signaling pathway via sponging miR-615-5p | [146] |
- Upregulation, +; Downregulation, -; Flap endonuclease 1, FEN1; Temozolomide, TMZ; Thyroid hormone receptor interactor 13, TRIP13.
3.2.4 Autophagy
Autophagy is a highly conserved cellular process of intracellular lysosomal degradation and organelle recycling controlled by more than 40 autophagy-related genes [148, 149]. Autophagy can be categorized as at least three distinct forms: macroautophagy [150], microautophagy (wrapping and degrading cytoplasmic components by bending and folding inwards of lysosomal membranes) [151], and chaperone-mediated autophagy (direct transport of misfolded proteins recognized by translocons into lysosomes) [152]. The most typical type of autophagy is macroautophagy, so the term “autophagy” is often used to refer to macroautophagy. Autophagy plays roles in health and disease states, such as embryonic development [153] and neurodegenerative diseases [154]. Notably, autophagy is involved in cancer progression. Intriguingly, autophagy can exert both pro- and anticancer effects. Taking cancer promotion as an example, when cells are starved, autophagy is enhanced to meet the energy and material needs of cancer cells. The neighbor of BRCA1 gene 1 (NBR1), an autophagy-related receptor, could steer MHC I from the surface of cancer cells into the cytoplasm. As a result, autophagy can reduce the expression level of MHC I on the surface of cancer cells, thereby impeding antigen presentation [155]. In mouse models, blocking the autophagy process restored the expression level of MHC I on the surface of cancer cells, which in turn enhanced antigen presentation and thus, weaken the immune escape of cancer cells [156].
As a “junk cleaner”, the autophagy process is also detected in OSCC. Elevated expression level of LC3II (an autophagy-related gene) was observed in OSCC, whereas migration and invasion of OSCC cells were silenced by autophagy gene blockers [157]. It is well known that ncRNAs have been shown to function as cancer suppressors or oncogenes in OSCC progression. Then, whether autophagy and ncRNAs have synergistic or antagonistic effects in OSCC has attracted the attention of scholars. Gao et al. [158] found that hypoxia enhanced both the expression level of circCDR1as and the autophagy process in OSCC cells. Mechanistically, circCDR1as facilitated the autophagy process via targeting multiple key regulators. Meanwhile, circCDR1as enhanced the autophagy process in OSCC cells by sponging miR-671-5p, inhibiting mTOR, and upregulating AKT and ERK signaling pathways. Thus, ncRNAs serve as upstream messengers along signaling pathways responsible for the regulation of OSCC by autophagy, indicating that targeting ncRNAs could alleviate the autophagy-related OSCC process (Figure 4). However, it remains largely unknown as to how autophagy is regulated by other ncRNAs in OSCC.
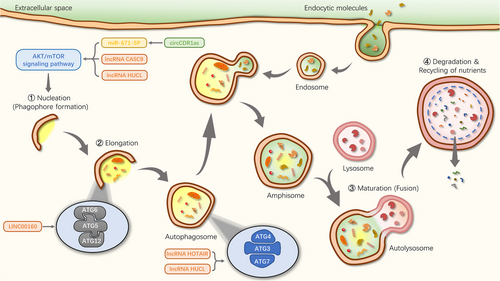
Autophagy and ncRNAs are not only involved in cancer progression, but in recent years the two have been found to jointly regulate the development of cancer drug resistance (Table 5). For example, miR-519a enhanced TMZ-induced autophagy and apoptosis processes, while inhibition of miR-519a decreased cellular autophagy and promoted TMZ resistance. In vivo, miR-519a sensitized cells to TMZ and enhanced apoptosis by boosting the cellular autophagy process [159]. In OSCC, autophagic flux was higher in drug-resistant cells compared to parental cells, and inhibition of autophagy led to decreased stemness, suggesting that autophagy enhanced CDDP-induced stemness and chemoresistance in OSCC cells [79]. Interestingly, ncRNAs are also implicated in the autophagy-mediated drug-resistant process in OSCC cells. The expression levels of ATG3 and ATG7 were reduced after lncRNA HOTAIR blocking, which inhibited autophagy. In parallel, the rate of apoptosis increased and the sensitivity of OSCC cells to CDDP was enhanced [67]. Ectopic expression of lncRNA highly upregulated in liver cancer (HULC) induced autophagy process in cancer cells, while lncRNA HULC silencing sensitized cancer cells to anti-tumor drugs by inhibiting the autophagy process. Importantly, lncRNA HULC was aberrantly upregulated in OSCC cell lines compared to normal cells and inhibition of lncRNA HULC expression suppressed cancer cell proliferation and drug resistance [160, 161]. Thus, inhibition of lncRNA HULC to alleviate OSCC resistance may be partially mediated by the autophagic signaling pathways. Collectively, inhibition of the autophagy process modulated by expression levels of ncRNAs can impair resistance of OSCC cells to chemotherapeutic drugs, thereby, promoting apoptosis and alleviating OSCC progression. Autophagy inhibitors may be promising adjuvant approaches in the fight against the drug resistance of OSCC. Therefore, there is an urgent need to develop new autophagy modulators with higher efficacy and lower toxicity for the treatment of drug resistance of OSCC.
| ncRNA | Expression | Sample(s) | Mechanism in autophagy | Reference |
|---|---|---|---|---|
| circCDR1as | + | OSCC tissues; Tca-8113, SCC-15, and HOK cells | Sponging miR-671-5p, inhibiting mTOR and upregulating AKT and ERK½ signaling pathways | [158] |
| miR-519a | - | U87-MG cells (glioblastoma) | Enhancing chemosensitivity and promoting autophagy by targeting STAT3/Bcl2 signaling pathway | [159] |
| lncRNA HOTAIR | + | CAL-27 cells | Upregulating expression of MAP1LC3B, beclin1, ATG3 and ATG7 | [67] |
| lncRNA HULC | + | OSCC tissues; SCC-9, SCC-15, SCC-25 and CAL-27 cells | Enhancing EMT process | [161] |
| lncRNA CASC9 | + | OSCC tissues; SCC-15 and CAL-27 cells | Enhancing AKT/mTOR signaling pathway | [162] |
| linc00160 | + | HCC tissues; MHCC-97, HCCLM-3, Hep-3B, and Huh-7 cells | Promoting autophagy and drug resistance by regulating miR-132-targeted PIK3R3 | [163] |
- Upregulation, +; Downregulation, -; Hepatocellular carcinoma, HCC; Microtubule-associated protein 1 light chain 3B, MAP1LC3B; Phosphoinositide-3-kinase regulatory subunit 3, PIK3R3.
In general, with the progression of chemotherapeutic drug action on cancer cells and conditions of cancer cells, drug resistance mechanisms can be mainly attributed to drug efflux mediated by transmembrane transporter proteins, elevated apoptosis threshold induced by EMT, enhanced DNA repair capacity, and drug degradation due to autophagy process. Certainly, ncRNAs can also regulate the OSCC resistance process in other ways besides the aforementioned drug-resistant mechanisms. The glycolytic pathways can also be mediated by ncRNAs to trigger drug resistance of OSCC. Wang et al. [164] found that lnc-p23154 promoted Glut1 expression, triggered glycolytic dysregulation, and induced drug resistance of OSCC by directly targeting the 3'UTR of miR-378a-3p. Importantly, the mechanisms of different drug resistance of OSCC appear to be independent, but in fact the mechanisms interact with each other. For example, EMT-TFs could recognize ABC transporter genes to regulate drug efflux, and the BRCA1 gene is involved in both DNA damage repair and the autophagy process. At the same time, ncRNAs play connecting roles like bridges. Consequently, a better understanding of the mechanisms of ncRNA-mediated drug resistance of OSCC can improve the basis for developing approaches to target ncRNAs to alleviate OSCC resistance.
4 NCRNA-CENTERED APPROACHES TO MITIGATE DRUG-RESISTANCE OF OSCC
Exploring the mechanisms of ncRNA-mediated drug resistance of OSCC could allow us to discover novel ways of attenuating or blocking drug-resistant processes. Many approaches have shown success in rescuing chemotherapeutic agents from acquired or intrinsic drug resistance. Currently, major approaches for targeting ncRNAs to alleviate drug resistance of OSCC include targeting oral cancer stem cells, the use of adjuvant drugs, and interfering with signaling pathways.
4.1 Targeting oral cancer stem cells
Oral cancer stem cells (OCSCs) are capable of self-renewal within a long period of time and reproduce the different cell lineages found in the primary cancers [165, 166]. One of the characteristics of OCSCs is the development of drug resistance in cancer cells, including conventional chemotherapies and immunotherapies. Therefore, OCSC-targeted therapies are promising treatment approaches to overcome the drug resistance of OSCC. Among miRNA families, let-7c is widely viewed as a tumor suppressor. The expression level of let-7c was found downregulated in OCSCs, while let-7c overexpression weakened stemness hallmarks and reversed chemoresistance [167]. Moreover, let-7c overexpression inhibited IL-8 secretion, suggesting that the upregulation of let-7c could weaken the stemness of OCSCs, thereby, enhancing the cytotoxicity of CDDP [168]. The expression level of CD133 was found elevated in OCSCs and drug resistance was enhanced. Notably, combination therapies with targeting CD133 and administrating CDDP inhibited OCSC-mediated OSCC initiation [169]. Dysregulated expression level of the transcription factor SOX2 regulated drug resistance of cancer cells to existing cancer therapies [170]. The expression level of SOX2 was upregulated in OSCC, and OCSC property was strengthened. SOX2 silencing suppressed expression levels of drug-resistant genes in OCSCs. Meanwhile, the knockdown of SOX2 combining with CDDP treatment attenuated drug resistance and improved the survival of OSCC mice [171]. Natural compound honokiol diminished self-renewal of OCSCs. At the same time, honokiol potentiated the effect of CDDP and inhibited cancer stemness of OCSCs, suggesting that honokiol may be an adjunct to the treatment of OSCC [172]. Taken together, to alleviate drug resistance of OSCC, it is essential to improve knowledge on OCSCs, with a particular focus on molecular features.
4.2 Applying adjuvant drugs
Combinations of multiple drugs have also been used to induce apoptosis in drug-resistant cells via enhancing cytotoxicity. Aldo-keto reductase (AKR) 1C family has been found to be associated with drug resistance [173]. AKR1Cs (including AKR1C1, AKR1C2, AKR1C3, and AKR1C4) were shown to be upregulated in CDDP-resistant OSCC cells. Mefenamic acid, an inhibitor of AKR1Cs, restored the sensitivity of drug-resistant cells to CDDP and 5-FU [174]. Isomahanine was able to induce endoplasmic reticulum stress in drug-resistant OSCC cells, ultimately inducing apoptosis [175]. Moreover, ursolic acid (UA) inhibited the phosphorylation of the AKT/BAD signaling pathway in drug-resistant OSCC cells, which in turn activated intrinsic apoptotic mechanisms [176]. Additionally, UA attenuated cancer cell stemness and thus reversed chemoresistance by interfering with miR-149-5p [177]. As such, drugs targeting ncRNAs hold potential in alleviating the drug resistance of OSCC. However, it is unknown whether the effect of these drugs in combination with first-line chemotherapeutic agents will amplify side effects in patients. Hence, this provides a direction for future exploration of multiple combination therapies.
4.3 Interfering with drug resistance-associated signaling pathways
Targeted inhibition of EGFR and related signaling pathways has been used as a treatment option for cancers. EGFR and its downstream signaling pathways were confirmed to be associated with CDDP sensitivity. EGFR inhibitors sensitized OSCC cells to 5-FU and CDDP. It has been proposed that inhibition of the EGFR signaling pathway may serve as a reasonable strategy for the treatment of drug-resistant OSCC patients [178, 179]. Furthermore, Li et al. [180] detected a higher expression level of β-catenin in CDDP-treated OSCC cells compared to controls. The sensitivity of OSCC cells to CDDP was enhanced by β-catenin silencing. In addition, the Wnt signaling pathway was also inhibited by β-catenin silencing. This indicates that blocking the Wnt/β-catenin signaling pathway could reverse drug resistance of OSCC cells. A variety of ncRNAs are known to be involved in the Wnt/β-catenin signaling pathway, including miR-106a and lncRNA placenta‑specific protein 2 (PLAC2) [181, 182]. On the other hand, it has been suggested that vitamin D can reduce the risk of many cancers. Also, in OSCC, vitamin D sensitized cancer cells to CDDP. Mechanistically, vitamin D inhibited the activation of the NF-κB signaling pathway, thereby enhancing CDDP toxicity in OSCC cells [183]. It is well established that ncRNAs and NF-κB signaling pathway are involved in OSCC process while it is unclear whether there are ncRNAs which regulate vitamin D-mediated inhibition of NF-κB signaling pathway. Therefore, interfering with drug-resistant signaling pathways mediated by ncRNAs can be an intervention to reverse the drug resistance of OSCC and enhance chemotherapeutic drug toxicity.
At the current stage, the development of ncRNA-based therapeutic approaches for drug resistance of OSCC has been correspondingly successful. However, how to modify ncRNAs to enhance the toxicity of chemotherapeutic drugs still needs to be further explored, especially in combination with emerging technologies.
5 FUTURE PERSPECTIVES
The systemic toxicity and low bioavailability of chemotherapeutic agents are current challenges in the treatment of OSCC. Hence, the development of advanced drug delivery strategies is urgently needed (Figure 5). It has been established that the toxic effects of CDDP on cancer cells can be scavenged by GSH catalyzed by GST (GSH-S-transferase). Therefore, it can be hypothesized that downregulated GST could restore OSCC cell apoptosis induced by CDDP. Based on the above view, Han et al. [184] constructed a GST inhibitor [ethacrynic acid (ECA)]-loadable nanomaterial termed MPEG-PLA-SS-ECA, and modified into nanoparticles carrying pingyangmycin and carboplatin. ECA, pingyangmycin, and carboplatin could all be released uniformly. In their results, MPEG-PLA-SS-ECA nanoparticles were shown to restore the chemosensitivity of drug-resistant OSCC cell lines. Meanwhile, Yang et al. [185] also developed Pt(IV)-NPs assembled from biotin-labeled Pt(IV) prodrug derivative and cyclodextrin-functionalized IR780. Since IR780 acted as a targeting ligand for mitochondria, Pt(IV)-NPs could localize in mitochondria and release CDDP, inducing mitochondrial DNA damage, thereby, downregulating GSH level and inhibiting DNA repair mechanisms. Furthermore, a new drug delivery system (FA-PEG-S-S-PCL@PTX, FA-NPs) was able to deliver PTX, inhibit GSH level, effectively mitigating OSCC progression [186]. In addition to interfering with GSH level, nano-delivery platforms that blocked the tumor cell cycle have been developed. Synthesized polyethylene glycol-graphene quantum dots-Pt (GPt) sensitized OSCC cells to chemotherapeutic agents, ultimately blocking the S-phase cell cycle and promoting apoptosis of OSCC cells [187]. The coloaded high-density lipoprotein-mimicking nanoparticles (HMNs) comprising NLS-Dox/anti-miR21 restored drug sensitivity in cancer cells with greater cytotoxicity [188]. Considering that miR-21 was also involved in the mechanisms of OSCC resistance, it could be speculated that HMNs could reverse the drug resistance of OSCC. Direct modification of ncRNAs, instead of loading ncRNAs, has also been proposed for targeting cancer genes. For example, siRNAs that chemically bind to carriers form carrier-siRNA conjugates, and lipid and PEG molecules modify siRNA to form self-assembled lipid nanoparticles [72]. Given the complex intracellular microenvironment, it is recommended that targeting ncRNAs with two or more different vectors improve drug delivery. Altogether, nanoparticles have shown superiority against tumor cell chemoresistance, and therefore, they can serve as a potential strategy against OSCC-resistant patients.
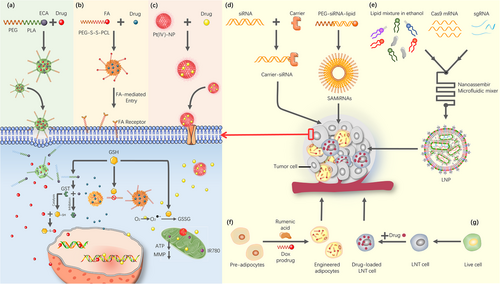
In recent years, CRISPR systems have also been expanded for application in tumor drug resistance therapy. On the one hand, key drivers of drug-resistant tumors can be screened by CRISPR systems. By using genome-wide CRISPR/Cas9 library screening, phosphoglycerate dehydrogenase (PHGDH) was identified as a key driver of sorafenib resistance in hepatocellular carcinoma, suggesting that PHGDH could serve as a target for alleviating sorafenib resistance [189]. This application could support precision medicine for tumor drug resistance. On the other hand, Rosenblum et al. [190] used lipid nanoparticles (LNP) as a delivery vehicle to encapsulate Cas9 mRNA and sgRNAs. As a result, this delivery system was validated to significantly inhibit tumor growth and increase survival rate by 80% in mouse models of glioblastoma and metastatic ovarian cancer. These strategies provided a clue to treating OSCC resistance by using CRISPR systems to screen for key drivers of OSCC resistance, followed by combining CRISPR systems with multiple technologies (like nanotechnology) to construct targeted drug delivery platforms. This concept will be the direction of future research.
Besides the two strategies mentioned above, the “self-cellular drug delivery” platform has recently acquired considerable attention. Both glucosamine and glucose were known to be recognized by glucose transporters on the surface of red blood cells. By conjugating insulin to glucosamine, the red blood cells thus served as the insulin-carrying van. Under high blood glucose levels, glucose competitively bound transporter proteins, resulting in insulin freeing and thus lowering blood glucose levels. Notably, red blood cells can be replaced by nanoparticles modified with glucose transporters, which will provide solutions for the construction of other bionic cells [191]. This system is a biocompatible “intelligent drug delivery system” that can autonomously regulate drug levels according to different conditions. Furthermore, rumenic acid (RA), an anticancer fatty acid, and Dox prodrug were enveloped in adipocytes. Modified adipocytes acted as a Trojan horse for anticancer drug delivery through lipid metabolism of tumor cells, thereby, enhancing drug transport efficiency [192]. Moreover, Ci et al. [193] used liquid nitrogen to prepare dead acute myeloid leukemia (AML) cells and constructed drug delivery vehicles that encapsulated Dox. Due to the fact that dead cells have similar protein expression as the source cells, dead cells kept their bone marrow homing capability of live AML cells. The results of in vivo experiments revealed that cryo-shocked cancer cells prolonged the blood half-life of drugs and improved enrichment of chemotherapeutic drugs in the bone marrow. In addition, the therapy significantly prolonged the survival of AML mice when combined with immune adjuvants.
Compared to drug delivery platforms constructed with synthetic nanomaterials, drug carriers based on self-cells maintained cellular targeting as well as good biocompatibility. However, the self-cellular drug delivery platform remains in initial stages, and it will be possible to modify dead cells carrying multiple anticancer drugs or to loading different drugs in different locations of the dead cells at later stages. Collectively, several platforms based on nano-delivery and self-cellular drug delivery hold promise for the treatment of many drug-resistant cancers, especially OSCC.
6 CONCLUSIONS
Growing evidence suggests the involvement of ncRNAs in drug resistance of OSCC. The important roles of ncRNAs in drug resistance make them potential targets for cancer therapies. This review highlighted the functions and mechanisms of ncRNAs such as miRNAs, lncRNAs, and circRNAs in the drug-resistant process of OSCC, and ultimately elucidated that treatment targeting these aberrantly expressed ncRNAs would be a promising approach to reverse drug resistance. Therapeutic interventions based on ncRNAs in combination with conventional chemotherapies may be an ideal option to address drug resistance in OSCC patients. However, it remains a challenge to screen out key ncRNAs from the large number of ncRNAs. Although ncRNAs have been extensively studied in OSCC, their roles as therapeutic targets for drug resistance of OSCC remains to be explored in depth. Moreover, studies have mainly focused on miRNAs and lncRNAs, and exploration of circRNA-mediated drug resistance of OSCC is still relatively rare. A new space for cancer drug resistance treatment has opened by the combination of multiple technologies for the development of chemotherapy drug delivery platforms, such as nanomaterials, genome editing, and modifying self-cells. Altogether, strategies are proposed to accelerate the pace from the lab to the bedside, and ncRNAs are expected to become novel targets for OSCC drug-resistant therapies.
DECLARATIONS
AUTHORS’ CONTRIBUTIONS
Manuscript writing (original draft): MX, LQY, YWY
Conceptualization/funding acquisition: XT, ZL
Manuscript writing, review, and editing: MX, WYR, CR, WL
Manuscript writing, vetting, and final approval: MX, LQY, YWY, WYR, CR, WL, XT, ZL
ACKNOWLEDGEMENTS
We thank XT and ZL for their scientific advice and critical reading of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
FUNDING
The present work was supported by the National Natural Science Foundation of China [Nos. 81700522]; the Natural Science Foundation of Anhui Province [1808085MH235, 1908085QH328]; the Grants for Scientific Research of BSKY from Anhui Medical University [XJ201706].
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




