A New Triterpene Glycoside from the Stems of Akebia quinata
Graphical Abstract
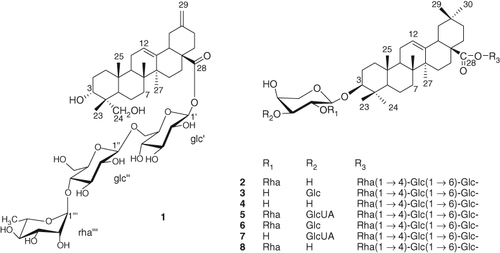
A new triterpene glycoside, named akeqintoside E, 3 αα,23αα-dihydroxy-30-norolean-12,20(29)-dien-28-oic acid 28-O-αα-L-rhamnopyranosyl-(1→4)-O-ββ-D-glucopyranosyl-(1→6)-O-ββ-D-glucopyranosyl ester (1), along with seven known compounds, akeboside Sth (2), begoniifolide A (3), akebia saponin PJ2
(4), 3ββ-[(O-GlcUA-(1–3)-O-[Rha-(1–2)]-Ara)oxy]olean-12-en-28-oic acid O-Rha-(1–4)-Glc-(1–6)-Glc ester (5), kalopanax saponin D (6), 3ββ-[(O-GlcUA-(1–3)-Ara)oxy]olean-12-en-28-oic acid O-Rha-(1–4)-Glc-(1–6)-Glc ester (7), and akebia saponin PJ3
(8), was isolated from Akebia quinata. The structures of the compounds were identified on the basis of 1D and 2D NMR, including 1H–
1H COSY, HSQC, HMBC, and NOESY spectroscopic analyses.