HFIP-Promoted E-selective Asymmetric Crotylboration of Quaternary α-Silyl Crotylboronate with Aldehydes
Ruiqi Tong
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorYan Zhang
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorShiyang Liu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorJiahui Gao
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorLiying Huang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorYi Li
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorLi Fu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorDr. Wanshu Wang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorProf. Dr. Lu Gao
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhishan Su
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
E-mail: [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenlei Song
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
E-mail: [email protected], [email protected]
Search for more papers by this authorRuiqi Tong
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorYan Zhang
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorShiyang Liu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorJiahui Gao
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorLiying Huang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorYi Li
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorLi Fu
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorDr. Wanshu Wang
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorProf. Dr. Lu Gao
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhishan Su
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
E-mail: [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenlei Song
Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced, Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, 610041 China
E-mail: [email protected], [email protected]
Search for more papers by this authorGraphical Abstract
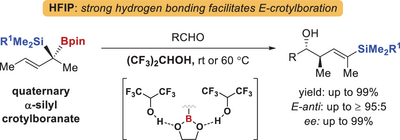
Hexafluoroisopropanol (HFIP)—a polar alcoholic solvent that has been underutilized in allylboration—enables otherwise challenging (E)-selective crotylboration of enantioenriched quaternary α-silyl crotylboronates with aldehydes. HFIP's strong hydrogen bonding effect increases the Lewis acidity of the boron center and the steric effect of Bpin, resulting in high yields and (E)-selectivity.
Abstract
Polyketide synthesis consistently requires the development of linchpin-type allylboron reagents, such as α-silyl crotylboronates. Traditionally, these reagents feature α-tertiary substitution and are used in aprotic solvents, typically yielding homoallylic alcohols with disubstituted (Z)-vinylsilanes. Here, we report the asymmetric synthesis of enantioenriched quaternary α-silyl crotylboronates via stereospecific allylic Matteson homologation. We further demonstrate that hexafluoroisopropanol (HFIP)—a polar alcoholic solvent that has been underutilized in allylboration—not only enhances reaction reactivity but also overrides the typical (Z)-selectivity, enabling access to the otherwise challenging (E)-selective products. The method enables the rapid construction of (E)-anti-2,4-dimethylpent-3-en-1-oxy motif, a structural unit frequently found in polyketides, and allows the protecting-group-free synthesis of PM050463. DFT calculations suggest that HFIP's strong hydrogen bonding effect increases the Lewis acidity of the boron center and the steric effect of Bpin, resulting in high yields and (E)-selectivity.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202508944-sup-0001-SupMat.pdf12.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Kobayashi, T. Kubota, J. Nat. Prod. 2007, 70, 451–460.
- 2H. Zhang, J. Zou, X. Yan, J. Chen, X. Cao, J. Wu, Y. Liu, T. Wang, Mar. Drugs 2021, 19, 180.
- 3L. L. Hong, Y. F. Ding, W. Zhang, H. W. Lin, Marine Life Sci. Technol. 2022, 4, 356–372.
- 4R. Das, A. Rauf, S. Mitra, T. B. Emran, M.d J. Hossain, Z. Khan, S. Naz, B. Ahmad, A. Meyyazhagan, K. Pushparaj, C. C. Wan, B. Balasubramanian, K. R.r. Rengasamy, J. Simal-Gandara, Chem. Interact. 2022, 365, 110072.
- 5S.-i. Shirokawa, M. Kamiyama, T. Nakamura, M. Okada, A. Nakazaki, S. Hosokawa, S. Kobayashi, J. Am. Chem. Soc. 2004, 126, 13604–13605.
- 6S. Hosokawa, K. Yokota, K. Imamura, Y. Suzuki, M. Kawarasaki, K. Tatsuta, Tetrahedron Lett. 2006, 47, 5415–5418.
- 7M. Shinoyama, S.-i. Shirokawa, A. Nakazaki, S. Kobayashi, Org. Lett. 2009, 11, 1277–1280.
- 8M. Yamaoka, A. Nakazaki, S. Kobayashi, Tetrahedron Lett. 2010, 51, 287–289.
- 9H. Sato, S. Hosokawa, Synlett 2019, 30, 577–580.
- 10J. A. Marshall, N. D. Adams, J. Org. Chem. 1998, 63, 3812–3813.
- 11J. A. Marshall, N. D. Adams, J. Org. Chem. 1999, 64, 5201–5204.
- 12J. A. Marshall, J. Org. Chem. 2007, 72, 8153–8166.
- 13B. M. Trost, J. P. N. Papillon, T. Nussbaumer, J. Am. Chem. Soc. 2005, 127, 17921–17937.
- 14B. M. Trost, W.-J. Bai, C. E. Stivala, C. Hohn, C. Poock, M. Heinrich, S. Xu, J. Rey, J. Am. Chem. Soc. 2018, 140, 17316–17326.
- 15S. Hanessian, J. Ma, W. Wang, J. Am. Chem. Soc. 2001, 123, 10200–10206.
- 16A. Fürstner, E. Kattnig, O. Lepage, J. Am. Chem. Soc. 2006, 128, 9194–9204.
- 17J. G. Geist, R. Barth, W. R. Roush, Org. Lett. 2013, 15, 58–61.
- 18S. Gao, M. Duan, Q. Shao, K. N. Houk, M. Chen, J. Am. Chem. Soc. 2020, 142, 18355–18368.
- 19S. Gao, M. Duan, J. Liu, P. Yu, K. N. Houk, M. Chen, Angew.Chem. Int. Ed. 2021, 60, 24096–24106.
- 20T. E. Smith, S. J. Fink, Z. G. Levine, K. A. McClelland, A. A. Zackheim, M. E. Daub, Org. Lett. 2012, 14, 1452–1455.
- 21M. Gravel, K. A. Thompson, M. Zak, C. Bérubé, D. G. Hall, J. Org. Chem. 2002, 67, 3–15.
- 22K. A. Scheidt, T. D. Bannister, A. Tasaka, M. D. Wendt, B. M. Savall, G. J. Fegley, W. R. Roush, J. Am. Chem. Soc. 2002, 124, 6981–6990.
- 23F. Kleinbeck, E. M. Carreira, Angew. Chem. Int. Ed. 2009, 48, 578–581.
- 24F. Kleinbeck, G. J. Fettes, L. D. Fader, E. M. Carreira, Chem. - Eur. J. 2012, 18, 3598–3610.
- 25X. Li, Q. L. Song, Org. Chem. Front. 2025, 12, 3129–3142.
- 26Y. Yamamoto, H. Yatagai, K. Maruyama, J. Am. Chem. Soc. 1981, 103, 3229–3231.
- 27D. J. S. Tsai, D. S. Matteson, Organometallics 1983, 2, 236–241.
- 28M. Shimizu, H. Kitagawa, T. Kurahashi, T. Hiyama, Angew. Chem. Int. Ed. 2001, 40, 4283–4286.
10.1002/1521-3773(20011119)40:22<4283::AID-ANIE4283>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 29L. Carosi, H. Lachance, D. G. Hall, Tetrahedron Lett. 2005, 46, 8981–8985.
- 30M. Chen, W. R. Roush, Org. Lett. 2013, 15, 1662–1665.
- 31J. Chen, S. Gao, M. Chen, Org. Lett. 2019, 21, 9893–9897.
- 32M. Y. Huang, Y. T. Zhao, C. D. Zhang, S. F. Zhu, Angew. Chem. Int. Ed. 2022, 61, e202203343.
- 33M.-Y. Huang, J.-B. Zhao, C.-D. Zhang, Y.-J. Zhou, Z.-S. Lu, S.-F. Zhu, J. Am. Chem. Soc. 2024, 146, 9871–9879.
- 34J. Kim, E. Lee, S. H. Cho, Asian J. Org. Chem. 2019, 8, 1664–1667.
- 35J. Park, Y. Jung, J. Kim, E. Lee, S. Y. Lee, S. H. Cho, Adv. Synth. Catal. 2021, 363, 2371–2376.
- 36Y. Jung, S. Y. Yoo, Y. Jin, J. You, S. Han, J. Yu, Y. Park, S. H. Cho, Angew. Chem. Int. Ed. 2023, 62, e202218794.
- 37S. J. T. Jonker, R. Jayarajan, T. Kireilis, M. Deliaval, L. Eriksson, K. J. Szabó, J. Am. Chem. Soc. 2020, 142, 21254–21259.
- 38N. Oyama, S. Akiyama, K. Kubota, T. Imamoto, H. Ito, Eur. J. Org. Chem. 2022, e202200664.
- 39I. Fujii, H. Hirata, H. Moniwa, R. Shintani, Chem. Commun. 2024, 60, 6921–6924.
- 40J. Lu, Z. Song, Y. Zhang, Z. Gan, H. Li, Angew. Chem. Int. Ed. 2012, 51, 5367–5370.
- 41L. Li, X. Ye, Y. Wu, L. Gao, Z. L. Song, Z. Yin, Y. Xu, Org. Lett. 2013, 15, 1068–1071.
- 42H. Li, H. Xie, Z. Zhang, Y. Xu, J. Lu, L. Gao, Z. L. Song, Chem. Commun. 2015, 51, 8484–8487.
- 43Y. Chu, Q. Pu, Z. Tang, L. Gao, Z. L. Song, Tetrahedron 2017, 73, 3707–3713.
- 44Z. Chu, K. Wang, L. Gao, Z. L. Song, Chem. Commun. 2017, 53, 3078–3081.
- 45Y. Zhang, Q. Guo, X. Sun, J. Lu, Y. Cao, Q. Pu, Z. Chu, L. Gao, Z. L. Song, Angew. Chem. Int. Ed. 2018, 57, 942–946.
- 46R. Q. Tong, S. Liu, C. Zhao, D. Y. Jiang, L. Gao, W. S. Wang, B. G. Ye, Z. L. Song, Org. Lett. 2022, 24, 7822–7827.
- 47M. Murakami, I. Usui, M. Hasegawa, T. Matsuda, J. Am. Chem. Soc. 2005, 127, 1366–1367.
- 48N. Xu, J. Xu, Q. Zhu, C. Liu, Adv. Synth. Catal. 2021, 363, 2403–2407.
- 49Z. Zhang, J. Liu, S. Gao, B. Su, M. Chen, J. Org. Chem. 2023, 88, 3288–3296.
- 50T. Liu, X.-R. Mao, S. Song, Z.-Y. Chen, Y. Wu, L.-P. Xu, P. Wang, Angew. Chem. Int. Ed. 2023, 62, e202216878.
- 51D. S. Matteson, D. Majumdar, Organometallics 1983, 2, 1529–1535.
- 52K. M. Sadhu, D. S. Matteson, Organometallics 1985, 4, 1687–1689.
- 53H. C. Brown, M. V. Rangaishenvi, S. Jayaraman, Organometallics 1992, 11, 1948–1954.
- 54M. Lombardo, S. Morganti, M. Tozzi, C. Trombini, Eur. J. Org. Chem. 2002, 2002, 2823.
- 55F. Peng, D. G. Hall, Tetrahedron Lett. 2007, 48, 3305–3309.
- 56L. Carosi, D. G. Hall, Angew. Chem. Int. Ed. 2007, 46, 5913–5915.
- 57F. Possémé, M. Deligny, F. Carreaux, B. Carboni, J. Org. Chem. 2007, 72, 984–989.
- 58A. Macé, F. Tripoteau, Q. Zhao, E. Gayon, E. Vrancken, J.-M. Campagne, B. Carboni, Org. Lett. 2013, 15, 906–909.
- 59B. A. Ondrusek, J. K. Park, D. T. McQuade, Synlett 2014, 25, 239–242.
- 60M. Brauns, F. Muller, D. Gülden, D. Böse, W. Frey, M. Breugst, J. Pietruszka, Angew. Chem. Int. Ed. 2016, 55, 1548–1552.
- 61M. Brauns, M. Mantel, J. Schmauck, M. Guder, M. Breugst, J. Pietruszka, Chem. - Eur. J. 2017, 23, 12136–12140.
- 62P. Ullrich, J. Schmauck, M. Brauns, M. Mantel, M. Breugst, J. Pietruszka, J. Org. Chem. 2020, 85, 1894–1905.
- 63 The crystallographic data for compound 2H-2b and 3x is available under accession number CCDC: 2408434 and CCDC: 2408435, and can be obtained free of charge from the Cambridge Crystallographic Data Centre via www. ccdc.cam.ac.uk/data_request/cif.
- 64For selected references of the phosphoric acid-catalyzed allylboration: P. Jain, J. C. Antilla, J. Am. Chem. Soc. 2010, 132, 11884–11886.
- 65T. Miura, Y. Nishida, M. Morimoto, M. Murakami, J. Am. Chem. Soc. 2013, 135, 11497–11500.
- 66C. A. Incerti-Pradillos, M. A. Kabeshov, A. V. Malkov, Angew. Chem. Int. Ed. 2013, 52, 5338–5341.
- 67T. Miura, J. Nakahashi, M. Murakami, Angew. Chem. Int. Ed. 2017, 56, 6989–6993.
- 68T. Miura, J. Nakahashi, W. Zhou, Y. Shiratori, S. G. Stewart, M. Murakami, J. Am. Chem. Soc. 2017, 139, 10903–10908.
- 69S. Gao, M. Chen, Org. Lett. 2018, 20, 6174–6177.
- 70B. E. Hetzler, G. Volpin, E. Vignoni, A. G. Petrovic, G. Proni, C. T. Hu, D. Trauner, Angew. Chem. Int. Ed. 2018, 57, 14276–14280.
- 71Y.-L. Zhang, Z.-N. Zhao, W.-L. Li, J.-J. Li, S. J. Kalita, U. Schneider, Y.-Y. Huang, Chem. Commun. 2020, 56, 10030–10033.
- 72S. Gao, M. Duan, K. N. Houk, M. Chen, Angew. Chem. Int. Ed. 2020, 59, 10540–10548.
- 73S. Gao, M. Chen, Org.Lett 2020, 22, 400–404.
- 74J. Chen, M. Chen, Org. Lett. 2020, 22, 7321–7326.
- 75J. Liu, M. Chen, Org.Lett 2020, 22, 8967–8972.
- 76For mechanical calculations of the phosphoric acid-catalyzed allylboration: M. N. Grayson, S. C. Pellegrinet, J. M. Goodman, J. Am. Chem. Soc. 2012, 134, 2716–2722.
- 77H. Wang, P. Jain, J. C. Antilla, K. N. Houk, J. Org. Chem. 2013, 78, 1208–1215.
- 78M. N. Grayson, Z. Yang, K. N. Houk, J. Am. Chem. Soc. 2017, 139, 7717–7720.
- 79 We also examined not only Lewis acids such as BF3·Et2O, Sc(OTf)3, Brönsted acids such as CF3CO2H but also Aggarwal's n-BuLi/TFAA condition, which were frequently-used to promote allylboration, but failed to facilitate allylboration nor promote E/Z selectivity.
- 80J. Liu, S. Gao, E. Miliordos, M. Chen, J. Am. Chem. Soc. 2023, 145, 19542–19553.
- 81M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery et al. Gaussian 09 (Revision D.01) I. Gaussian, Wallingford, CT, 2013.
- 82T. Lu, F. W. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 83W. Humphrey, A. Dalke, K. J. Schulten, Mol. Graphics. 1996, 14, 33–38.
- 84F. X. Tian, J. Qu, J. Org. Chem. 2022, 87, 1814–1829.
- 85H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood, J. Aubé, Chem. Rev. 2022, 122, 12544–12747.
- 86N. Zeidan, S. Bicic, R. J. Mayer, D. Lebœuf, J. Moran, Chem. Sci. 2022, 13, 8436–8443.
- 87J. Chen, W. Song, J. Yao, Z. Wu, Y.-M. Lee, Y. Wang, W. Nam, B. Wang, J. Am. Chem. Soc. 2023, 145, 5456–5466.
- 88N. V. Tzouras, L. P. Zorba, E. Kaplanai, N. Tsoureas, D. J. Nelson, S. P. Nolan, G. C. Vougioukalakis, ACS Catal. 2023, 13, 8845–8860.
- 89S. Cai, H. Tang, B. Li, Y. Shao, D. Zhang, H. Zheng,T. Qiao, X. Chu, G. He, X.-S. Xue, G. Chen, J. Am. Chem. Soc. 2024, 146, 5952–5963.
- 90T. A. To, N. T. A. Phan, B. K. Mai, T. V. Nguyen, Chem. Sci. 2024, 15, 7187–7197.
- 91S. E. Denmark, S. A. Tymonko, J. Am. Chem. Soc. 2005, 127, 8004–8005.
- 92Y. Zhang, J. S. Panek, Org. Lett. 2007, 9, 3141–3143.
- 93S. E. Denmark, J. H.-C. Liu, J. Am. Chem. Soc. 2007, 129, 3737–3744.
- 94S. E. Denmark, C. S. Regens, Acc. Chem. Res. 2008, 41, 1486–1499.
- 95T. Takeda, H. Wasa, A. Tsubouchi, Tetrahedron Lett. 2011, 52, 4575–4578.
- 96C. A. McAdam, M. G. McLaughlin, M. J. Cook, Org. Chem. Front. 2015, 2, 510–514.
- 97M. G. McLaughlin, C. A. McAdam, M. J. Cook, Org. Lett. 2015, 17, 10–13.
- 98A. Rivas, V. Pérez-Revenga, R. Alvarez, A. R. De Lera, Chem. - Eur. J. 2019, 25, 14399–14407.
- 99K. Miura, T. Hondo, S. Okajima, T. Nakagawa, T. Takahashi, A. Hosomi, J. Org. Chem. 2002, 67, 6082–6090.
- 100C. Schleissner, M. Pérez, A. Losada, P. Rodríguez, C. Crespo, P. Zúñiga, R. Fernández, F. Reyes, F. De La Calle, J. Nat. Prod. 2011, 74, 1590–1596.
- 101Y. Hayakawa, J. Saito, M. Izawa, K. Shin-ya, J. Antibiot. (Tokyo) 2014, 67, 831–834.
- 102H. Zhang, X. Zhang, Y. Huang, J. Yuan, X. Wei, J. Ju, J. Nat. Prod. 2022, 85, 625–633.
- 103H. Zhang, N. Gong, H. Zhang, Q. Li, J. Ma, X. Wei, W. Li, J. Ju, Org. Lett. 2022, 24, 9065–9070.
- 104W. S. Wadsworth, W. D. Emmons, J. Am. Chem. Soc. 1961, 83, 1733–1738.
- 105E. J. Corey, G. T. Kwiatkowski, J. Am. Chem. Soc. 1966, 88, 5654–5656.
- 106H. Nagaoka, Y. Kishi, Tetrahedron 1981, 37, 3873–3888.
- 107J. E. Moses, J. E. Baldwin, R. M. Adlington, Tetrahedron Lett. 2004, 45, 6447–6448.
- 108S. Hosokawa, K. Yokota, K. Imamura, Y. Suzuki, M. Kawarasaki, K. Tatsuta, Chemistry. 2008, 3, 1415–1421.
- 109B. H. Lipshutz, B. Amorelli, Tetrahedron Lett. 2009, 50, 2144–2146.