Chiral Spirobipyridine Synthesis by Cobalt-Catalyzed Enantioselective Double [2 + 2 + 2] Cycloaddition
Ke Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDanyang Zhu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorLuyu Cao
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Changkun Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
E-mail: [email protected]
Search for more papers by this authorKe Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorDanyang Zhu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorLuyu Cao
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Changkun Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
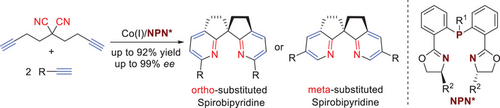
Chiral spirobiindanes are recognized as the privileged structures in the field of asymmetric catalysis. However, the structurally similar chiral spirobipyridines have not yet been explored as chiral ligands or organocatalysts due to the absence of efficient synthetic methods. Herein, we report a cobalt-catalyzed enantioselective synthesis of spirobipyridines via double [2 + 2 + 2] cycloaddition reaction. Spirobipyridines with ortho- or meta-substituents could be obtained with exclusive regioselectivity and up to 99% ee in the presence of cobalt and bisoxazolinephosphine ligands. Spirobipyridines coordinate with transition metals as chiral ligands. Spirobipyridine dioxides can be applied as chiral organocatalysts in the asymmetric allylation of aldehydes.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202504831-sup-0001-SuppMatS1.pdf18.3 MB | Supporting Information |
| anie202504831-sup-0002-SuppMatS2.zip1.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on chiral ligands with spiro skeleton see: S.-F. Zhu, Q.-L. Zhou, in Ligand Design in Metal Chemistry: Reactivity and Catalysis, 1st ed., (Eds.: M. Stradiotto, R. J. Lundgren), John Wiley & Sons, Ltd., Hoboken, NJ 2016.
- 2K. Ding, Z. Han, Z. Wang, Chem. Asian J. 2009, 4, 32–41.
- 3X. Hu, X. Zhang, W. Liu, Chin. J. Org. Chem. 2022, 42, 3102–3117.
- 4A. S. C. Chan, W.-H. Hu, C.-C. Pai, C.-P. Lau, Y. Jiang, A. Mi, M. Yan, J. Sun, R. Lou, J. Deng, J. Am. Chem. Soc. 1997, 119, 9570–9571.
- 5M. A. Arai, T. Arai, H. Sasai, Org. Lett. 1999, 1, 1795–1797.
- 6M. A. Arai, M. Kuraishi, T. Arai, H. Sasai, J. Am. Chem. Soc. 2001, 123, 2907–2908.
- 7For selected original papers see: J.-H. Zhang, J. Liao, X. Cui, K.-B. Yu, J. Zhu, J.-G. Deng, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, L. W. Chung, T. Ye, Tetrahedron: Asymmetry 2002, 13, 1363–1366.
- 8Y. Fu, J.-H. Xie, A.-G. Hu, H. Zhou, L.-X. Wang, Q.-L. Zhou, Chem. Commun. 2002, 480–481.
- 9J.-H. Xie, L.-X. Wang, Y. Fu, S.-F. Zhu, B.-M. Fan, H.-F. Duan, Q.-L. Zhou, J. Am. Chem. Soc. 2003, 125, 4404–4405.
- 10J.-H. Xie, X.-Y. Liu, J.-B. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 7329–7332.
- 11For personal accounts, see: J.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581–593.
- 12F. Yang, J.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2023, 56, 332–349.
- 13Z. Han, Z. Wang, X. Zhang, K. Ding, Angew. Chem. Int. Ed. 2009, 48, 5345–5349.
- 14X. Liu, Z. Han, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2014, 53, 1978–1982.
- 15Y. Ge, Z. Han, Z. Wang, C.-G. Feng, Q. Zhao, G.-Q. Lin, K. Ding, Angew. Chem. Int. Ed. 2018, 57, 13140–13144.
- 16Z. Freixa, M. S. Beentjes, G. D. Batema, C. B. Dieleman, G. P. F. van Strijdonk, J. N. H. Reek, P. C. J. Kamer, J. Fraanje, K. Goubitz, P. W. N. M. van Leeuwen, Angew. Chem. Int. Ed. 2003, 42, 1284–1287.
- 17X. Sala, E. J. G. Suárez, Z. Freixa, J. Benet-Buchholz, P. W. N. M. van Leeuwen, Eur. J. Org. Chem. 2008, 6197–6205.
- 18J. Li, G. Chen, Z. Wang, R. Zhang, X. Zhang, K. Ding, Chem. Sci. 2011, 2, 1141–1144.
- 19X. Wang, Z. Han, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2012, 51, 936–940.
- 20X. Wang, F. Meng, Y. Wang, Z. Han, Y.-J. Chen, L. Liu, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2012, 51, 9276–9282.
- 21X. Wang, P. Guo, Z. Han, X. Wang, Z. Wang, K. Ding, J. Am. Chem. Soc. 2014, 136, 405–411.
- 22J.-M. Tian, Y.-H. Yuan, Y.-Q. Tu, F.-M. Zhang, X.-B. Zhang, S.-H. Zhang, S.-H. Wang, X.-M. Zhang, Chem. Commun. 2015, 51, 9979–9982.
- 23J.-M. Tian, A.-F. Wang, J.-S. Yang, X.-J. Zhao, Y.-Q. Tu, S.-Y. Zhang, Z.-M. Chen, Angew. Chem. Int. Ed. 2019, 58, 11023–11027.
- 24J.-S. Yang, K. Lu, C.-X. Li, Z.-H. Zhao, F.-M. Zhang, X.-M. Zhang, Y.-Q. Tu, J. Am. Chem. Soc. 2023, 145, 22122–22134.
- 25G.-Q. Chen, B.-J. Lin, J.-M. Huang, L.-Y. Zhao, Q.-S. Chen, S.-P. Jia, Q. Yin, X. Zhang, J. Am. Chem. Soc. 2018, 140, 8064–8068.
- 26J. Huang, M. Hong, C.-C. Wang, S. Kramer, G.-Q. Lin, X.-W. Sun, J. Org. Chem. 2018, 83, 12838–12846.
- 27G.-Q. Chen, R. Xiao, X. Ding, J. Wang, B. Ma, Q. Lang, X. Zhang, Org. Lett. 2024, 26, 2811–2816.
- 28J. A. Varela, L. Castedo, C. Saá, Org. Lett. 1999, 1, 2141–2143.
- 29M. Claps, N. Parrinello, C. Saá, J. A. Varela, S. Caccamese, C. Rosini, Tetrahedron: Asymmetry 2006, 17, 1387–1393.
- 30A. Wada, K. Noguchi, M. Hirano, K. Tanaka, Org. Lett. 2007, 9, 1295–1298.
- 31J. Cai, L.-G. Bai, Y. Zhang, Z.-K. Wang, F. Yao, J.-H. Peng, W. Yan, Y. Wang, C. Zheng, W.-B. Liu, Chem 2021, 7, 799–811.
- 32W.-B. Liu, J.-H. Cai, CN 111646992A 2020.
- 33after our manuscript was submitted, Liu group reported a related and elegant enantioselective [2 + 2 + 2] spirobipyridine synthesis under nickel catalysis, see: L.-G. Bai, Y.-Q. Zheng, H.-N. Chen, J.-H. Cai, W.-B. Liu, J. Am. Chem. Soc. 2025, 147, 14574–14584.
- 34F. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677–8680.
- 35I. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373.
- 36C.-H. Xing, Y.-X. Liao, J. Ng, Q.-S. Hu, J. Org. Chem. 2011, 76, 4125–4131.
- 37B. Xu, S.-F. Zhu, X.-L. Xie, J.-J. Shen, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 11483–11486.
- 38X. Lin, L. Wang, Z. Han, Z. Chen, Chin. J. Chem. 2021, 39, 802–824.
- 39K. Li, M.-L. Li, Q. Zhang, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2018, 140, 7458–7461.
- 40T.-Y. Zhao, K. Li, L.-L. Yang, S.-F. Zhu, Q.-L. Zhou, Org. Lett. 2021, 23, 3814–3817.
- 41L.-L. Yang, D. Evans, B. Xu, W.-T. Li, M.-L. Li, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2020, 142, 12394–12399.
- 42A.-C. Han, L.-J. Xiao, Q.-L. Zhou, J. Am. Chem. Soc. 2024, 146, 5643–5649.
- 43J. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242–5245.
- 44H. Yang, R. Zhang, S.-Z. Zhang, Q. Gu, S.-L. You, ACS Catal. 2023, 13, 8838–8844.
- 45For other catalysts with ortho-substituents on SPINOL skeletons, see: D. Zhu, H.-Y. Luo, X.-S. Xue, Z.-M. Chen, Angew. Chem. Int. Ed. 2022, 61, e202211782.
- 46X.-Y. Zhang, D. Zhu, Y.-X. Huo, L.-L. Chen, Z.-M. Chen, Org. Lett. 2023, 25, 3445–3450.
- 47C. Xu, Y. Qi, X. Yang, X. Li, Z. Li, L. Bai, Org. Lett. 2021, 23, 2890–2894.
- 48M.-Y. Rong, J. Nie, S. Li, J.-A. Ma, Molecular Catal 2024, 552, 113679.
- 49K. Li, L. Wei, M. Sun, B. Li, M. Liu, C. Li, Angew. Chem. Int. Ed. 2021, 60, 20204–20209.
- 50For recent reviews on transition-metal-catalyzed pyridine synthesis see: T. Gläsel, B. N. Baumann, M. Hapke, Chem. Rec. 2021, 21, 3727–3745.
- 51J. Cai, K. Cen, W. Shen, L.-G. Bai, W.-B. Liu, Chem Catal 2022, 2, 2889–2897.
- 52K. Cen, M. Usman, W. Shen, M. Liu, R. Yang, J. Cai, Org. Biomol. Chem. 2022, 20, 7391–7404.
- 53K. Tanaka, in Comprehensive Chirality, 2nd ed., (Eds.: J. Cossy), Academic Press, Cambridge 2024.
10.1016/B978-0-32-390644-9.00038-X Google Scholar
- 54For the original works of bisoxazoline-phosphine (NPN*) ligands, see:Y. Jiang, Q. Jiang, G. Zhu, X. Zhang, Tetrahedron Lett. 1997, 38, 215–218.
- 55T. Yamagishi, M. Ohnuki, T. Kiyooka, D. Masui, K. Sato, M. Yamaguchi, Tetrahedron Asymm 2003, 14, 3275–3279.
- 56For Rh/NPN*-catalysed regio- and enantioselective allylation, see: W.-B. Xu, S. Ghorai, W. Huang, C. Li, ACS Catal. 2020, 10, 4491–4496.
- 57W.-Y. Huang, C.-H. Lu, S. Ghorai, B. Li, C. Li, J. Am. Chem. Soc. 2020, 142, 15276–15281.
- 58K. Li, C. Li, Org. Lett. 2020, 22, 9456–9461.
- 59M. Sun, M. Liu, C. Li, Chem. - Eur. J. 2021, 27, 3457–3462.
- 60M. Liu, H. Zhao, C. Li, Chin. Chem. Lett. 2021, 32, 385–388.
- 61W.-B. Xu, M. Sun, M. Shu, C. Li, J. Am. Chem. Soc. 2021, 143, 8255–8260.
- 62B. Li, M. Liu, S. U. Rehman, C. Li, J. Am. Chem. Soc. 2022, 144, 2893–2898.
- 63M. Sun, L. Wei, C. Li, J. Am. Chem. Soc. 2023, 145, 3897–3902.
- 64B. Li, Y. Luo, M. Liu, Y. Xia, C. Li, ACS Catal. 2023, 13, 5482–5490.
- 65For other reactions with NPN* ligands, see: J.-F. Chen, C. Li, Org. Lett. 2020, 22, 4686–4691.
- 66V. U. B. Rao, C. Wang, D. P. Demarque, C. Grassin, F. Otte, C. Merten, C. Strohmann, C. C. J. A. Loh, Nat. Chem. 2023, 15, 424–435.
- 67L. Qi, Q.-Q. Pan, X.-X. Wei, X. Pang, Z. Liu, X.-Z. Shu, J. Am. Chem. Soc. 2023, 145, 13008–13014.
- 68X. Wu, H. Xia, C. Gao, B. Luan, L. Wu, C. Zhang, D. Yang, L. Hou, N. Liu, T. Xia, H. Li, J. Qu, Y. Chen, Nat. Chem. 2024, 16, 398–407.
- 69C. Lin, J. Zhang, Z. Sun, Y. Guo, Q. Chong, Z. Zhang, F. Meng, Angew. Chem. Int. Ed. 2024, 63, e202405290.
- 70 For the details about the influence of ligand substituent on the regioselectivity, see supporting information.
- 71 Deposition numbers 2403732 (for 4n), 2289864 (for 6a), 2403736 (for 8e), 2403739 (for 19-TFA), 2403737 (for CuPF6-8e′), 2403729 (for Fe(OTf)2-6a•H2O) and CoI-L1 (2442749) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service, http://www.ccdc.cam.ac.uk/structures.
- 72I. Colomer, A. E. R. Chamberlain, M. B. Haughey, T. J. Donohoe, Nat. Rev. Chem. 2017, 1, 0088.
- 73H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood IV, J. Aubé, Chem. Rev. 2022, 122, 12544–12747.
- 74S. Ghosh, K. Patra, M. Baidya, Eur. J. Org. Chem. 2024, 27, e202301321. For further discussion on the effect of HFIP, see Supporting information.
- 75A. V. Malkov, P. C. Kočovsky, Eur. J. Org. Chem. 2007, 2007, 29–36.
- 76J. Fu, S. Fujimori, S. E. Denmark, in Lewis Base Catalysis in Organic Synthesis, (Eds: E. Vedejs, S. E. Denmark), Wiley-VCH, Weinheim 2016.
- 77A. V. Malkov, P. Kočovsky, in Lewis Base Catalysis in Organic Synthesis, (Eds: E. Vedejs, S. E. Denmark), Wiley-VCH, Weinheim 2016.
- 78Z.-Y. Liu, Z.-H. Wen, X.-C. Wang, Angew. Chem. Int. Ed. 2017, 56, 5817–5820.