Self-Compartmented Electrolyte Design for Stable Cycling of Lithium Metal Batteries under Extreme Conditions
Yu Ou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorDa Zhu
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorPan Zhou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorChangjian Li
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYang Lu
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorQingbin Cao
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorXuan Song
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorWenhui Hou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorShuaishuai Yan
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYingchun Xia
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorHangyu Zhou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
China Academy of Safety Science and Technology, Beijing, 100012 China
Search for more papers by this authorWeili Zhang
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorQingqing Feng
Tsinghua University Hefei Institute for Public Safety Research, Anhui, 230601 China
Search for more papers by this authorHong Xu
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Kai Liu
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]
Search for more papers by this authorYu Ou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorDa Zhu
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorPan Zhou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorChangjian Li
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYang Lu
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorQingbin Cao
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorXuan Song
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorWenhui Hou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorShuaishuai Yan
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYingchun Xia
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorHangyu Zhou
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
China Academy of Safety Science and Technology, Beijing, 100012 China
Search for more papers by this authorWeili Zhang
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorQingqing Feng
Tsinghua University Hefei Institute for Public Safety Research, Anhui, 230601 China
Search for more papers by this authorHong Xu
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Kai Liu
Department of Chemical Engineering, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
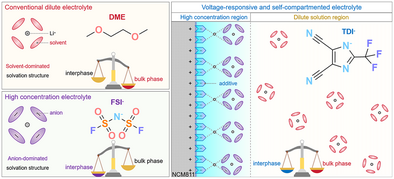
Self-compartmented electrolyte: A novel electrolyte concept utilizing LiTDI enables a high-concentration, anion-rich interfacial region and a low-concentration bulk electrolyte. This unique design simultaneously enhances interfacial stability and ion transport kinetics in high-voltage lithium-metal batteries.
Abstract
Electrolyte is the key component dictating lithium battery performance, especially under extreme conditions such as fast cycling and low temperatures. However, conventional electrolyte design principles, which generally rely on a homogeneous mixture of solvents, salts, and functional additives, fail to simultaneously meet the requirements for both anodic/cathodic interfacial stability and bulk ion-transport kinetics in lithium metal batteries. Herein, we present a self-compartmented electrolyte design methodology. Lithium 4,5-dicyano-2-(trifluoromethyl)imidazol-1-ide (LiTDI), featuring the ability to selectively self-assemble on the cathode/electrolyte interface, compartmented the electrolyte into a heterogonous one. Close to the cathode side, LiTDI could induce an interfacial high-concentration region, where the anion-rich solvation structure facilitates the formation of a stable cathode–electrolyte interphase (CEI). In the bulk, the electrolyte maintains a low concentration with low viscosity, ensuring fast ion transport and superior rate performance. Li||NCM811 cells achieve over 500 stable cycles with 80.3% capacity retention and deliver 169.3 mAh g−1 at a 10C discharge rate. Under low-temperature conditions (−20 °C), the cells maintained outstanding stability over 700 cycles at 0.5C charge/discharge, achieving capacity retention of 96.6% and an average Coulombic efficiency of 99.2%. This work provides a new electrolyte design paradigm, addressing the critical challenges of LMBs for high-voltage and low-temperature applications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202504632-sup-0001-SuppMat.pdf4.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. Zhang, T. Deng, S. Zhang, C. Wang, L. Chen, C. Wang, X. Fan, Adv. Mater. 2022, 34, 2107899.
- 2Y. Ou, P. Zhou, W. Hou, X. Ma, X. Song, S. Yan, Y. Lu, K. Liu, J. Energy Chem. 2024, 94, 360–392.
- 3J. Holoubek, H. Liu, Z. Wu, Y. Yin, X. Xing, G. Cai, S. Yu, H. Zhou, T. A. Pascal, Z. Chen, P. Liu, Nat. Energy 2021, 6, 303–313.
- 4S. Wang, J. Shi, Z. Liu, Y. Xia, Adv. Energy Mater. 2024, 14, 2401526.
- 5A.-M. Li, Z. Wang, T. Lee, N. Zhang, T. Li, W. Zhang, C. Jayawardana, M. Yeddala, B. L. Lucht, C. Wang, Nat. Energy 2024, 9, 1551–1560.
- 6J. Xiang, Y.-C. Lu, ACS Nano 2024, 18, 10726–10737.
- 7J. Qian, W. A. Henderson, W. Xu, P. Bhattacharya, M. Engelhard, O. Borodin, J.-G. Zhang, Nat. Commun. 2015, 6, 6362.
- 8S. Chen, J. Zheng, D. Mei, K. S. Han, M. H. Engelhard, W. Zhao, W. Xu, J. Liu, J. G. Zhang, Adv. Mater. 2018, 30, e1706102.
- 9C. M. Efaw, Q. Wu, N. Gao, Y. Zhang, H. Zhu, K. Gering, M. F. Hurley, H. Xiong, E. Hu, X. Cao, W. Xu, J.-G. Zhang, E. J. Dufek, J. Xiao, X.-Q. Yang, J. Liu, Y. Qi, B. Li, Nat. Mater. 2023, 22, 1531–1539.
- 10J. Holoubek, K. Kim, Y. Yin, Z. Wu, H. Liu, M. Li, A. Chen, H. Gao, G. Cai, T. A. Pascal, P. Liu, Z. Chen, Energy Environ. Sci. 2022, 15, 1647–1658.
- 11Z. Li, H. Rao, R. Atwi, B. M. Sivakumar, B. Gwalani, S. Gray, K. S. Han, T. A. Everett, T. A. Ajantiwalay, V. Murugesan, N. N. Rajput, V. G. Pol, Nat. Commun. 2023, 14, 868.
- 12Y. Yin, J. Holoubek, K. Kim, A. Liu, B. Bhamwala, S. Wang, B. Lu, K. Yu, H. Gao, M. Li, G. Raghavendran, G. Cai, W. Li, P. Liu, Y. S. Meng, Z. Chen, Angew. Chem. Int. Ed. 2024, e202420411.
- 13A.-M. Li, O. Borodin, T. P. Pollard, W. Zhang, N. Zhang, S. Tan, F. Chen, C. Jayawardana, B. L. Lucht, E. Hu, X.-Q. Yang, C. Wang, Nat. Chem. 2024, 16, 922–929.
- 14Z. Yu, H. Wang, X. Kong, W. Huang, Y. Tsao, D. G. Mackanic, K. Wang, X. Wang, W. Huang, S. Choudhury, Y. Zheng, C. V. Amanchukwu, S. T. Hung, Y. Ma, E. G. Lomeli, J. Qin, Y. Cui, Z. Bao, Nat. Energy 2020, 5, 526–533.
- 15Z. Yu, P. E. Rudnicki, Z. Zhang, Z. Huang, H. Celik, S. T. Oyakhire, Y. Chen, X. Kong, S. C. Kim, X. Xiao, H. Wang, Y. Zheng, G. A. Kamat, M. S. Kim, S. F. Bent, J. Qin, Y. Cui, Z. Bao, Nat. Energy 2022, 7, 94–106.
- 16S. C. Kim, J. Wang, R. Xu, P. Zhang, Y. Chen, Z. Huang, Y. Yang, Z. Yu, S. T. Oyakhire, W. Zhang, L. C. Greenburg, M. S. Kim, D. T. Boyle, P. Sayavong, Y. Ye, J. Qin, Z. Bao, Y. Cui, Nat. Energy 2023, 8, 814–826.
- 17T. Zhou, Y. Zhao, M. El Kazzi, J. W. Choi, A. Coskun, Angew. Chem. Int. Ed. 2022, 61, e202115884.
- 18Y. Zhao, T. Zhou, D. Baster, M. El Kazzi, J. W. Choi, A. Coskun, ACS Energy Lett. 2023, 8, 3180–3187.
- 19K. Lee, S.-H. Kwon, J. Kim, E. Park, I. Kim, H. C. Ahn, A. Coskun, J. W. Choi, ACS Energy Lett. 2024, 9, 2201–2211.
- 20X. Fan, X. Ji, L. Chen, J. Chen, T. Deng, F. Han, J. Yue, N. Piao, R. Wang, X. Zhou, X. Xiao, L. Chen, C. Wang, Nat. Energy 2019, 4, 882–890.
- 21D. Lu, R. Li, M. M. Rahman, P. Yu, L. Lv, S. Yang, Y. Huang, C. Sun, S. Zhang, H. Zhang, J. Zhang, X. Xiao, T. Deng, L. Fan, L. Chen, J. Wang, E. Hu, C. Wang, X. Fan, Nature 2024, 627, 101–107.
- 22M. Armand, P. Johansson, M. Bukowska, P. Szczeciński, L. Niedzicki, M. Marcinek, M. Dranka, J. Zachara, G. Żukowska, M. Marczewski, G. Schmidt, W. Wieczorek, J. Electrochem. Soc. 2020, 167.
- 23C. L. Berhaut, P. Porion, L. Timperman, G. Schmidt, D. Lemordant, M. Anouti, Electrochim. Acta 2015, 180, 778–787.
- 24C. L. Berhaut, R. Dedryvère, L. Timperman, G. Schmidt, D. Lemordant, M. Anouti, Electrochim. Acta 2019, 305, 534–546.
- 25I. A. Shkrob, K. Z. Pupek, J. A. Gilbert, S. E. Trask, D. P. Abraham, J. Phys. Chem. C 2016, 120, 28463–28471.
- 26L. Niedzicki, M. Kasprzyk, K. Kuziak, G. Z. Żukowska, M. Marcinek, W. Wieczorek, M. Armand, J. Power Sources 2011, 196, 1386–1391.
- 27C. Xu, S. Renault, M. Ebadi, Z. Wang, E. Björklund, D. Guyomard, D. Brandell, K. Edström, T. Gustafsson, Chem. Mater. 2017, 29, 2254–2263.
- 28R. Pan, Z. Cui, M. Yi, Q. Xie, A. Manthiram, Adv. Energy Mater. 2022, 12, 2103806.
- 29Y. Zhao, T. Zhou, M. Mensi, J. W. Choi, A. Coskun, Nat. Commun. 2023, 14, 299.
- 30M. Kim, J. An, S.-J. Shin, I. Hwang, J. Lee, Y. Park, J. Kim, E. Park, J. Kim, G. Park, S. Kim, A. Coskun, J. W. Choi, Energy Environ. Sci. 2024, 17, 6079–6090.
- 31Y. C. Xia, P. Zhou, X. Kong, J. K. Tian, W. L. Zhang, S. S. Yan, W. H. Hou, H. Y. Zhou, H. Dong, X. X. Chen, P. C. Wang, Z. Xu, L. Wan, B. G. Wang, K. Liu, Nat. Energy 2023, 8, 934–945.
- 32Y. Lu, Q. B. Cao, W. L. Zhang, T. Y. Zeng, Y. Ou, S. S. Yan, H. Liu, X. Song, H. Y. Zhou, W. H. Hou, P. Zhou, N. Hu, Q. Q. Feng, Y. Li, K. Liu, Nat. Energy 2025, 10, 191–204.
- 33Y. Ou, W. Hou, D. Zhu, C. Li, P. Zhou, X. Song, Y. Xia, Y. Lu, S. Yan, H. Zhou, Q. Cao, H. Zhou, H. Liu, X. Ma, Z. Liu, H. Xu, K. Liu, Energy Environ. Sci. 2025, 18, 1464–1476.
- 34B. Ma, H. Zhang, R. Li, S. Zhang, L. Chen, T. Zhou, J. Wang, R. Zhang, S. Ding, X. Xiao, T. Deng, L. Chen, X. Fan, Nat. Chem. 2024, 16, 1427–1435.
- 35W.-h. Hou, Y. Ou, T. Zeng, Q. Feng, Q. Cao, P. Zhou, Y. Xia, X. Song, W. Zhang, Y. Lu, S. Yan, H.-y. Zhou, H. Zhou, H. Liu, F. Liu, K. Liu, Energy Environ. Sci. 2024, 17, 8325–8336.
- 36Y. C. Xia, W. H. Hou, P. Zhou, Y. Ou, G. Y. Cheng, C. Guo, F. X. Liu, W. L. Zhang, S. S. Yan, Y. Lu, Y. X. Zeng, K. Liu, Nano Lett. 2024, 24, 12791–12798.
- 37M. S. Kim, Z. Zhang, P. E. Rudnicki, Z. Yu, J. Wang, H. Wang, S. T. Oyakhire, Y. Chen, S. C. Kim, W. Zhang, D. T. Boyle, X. Kong, R. Xu, Z. Huang, W. Huang, S. F. Bent, L.-W. Wang, J. Qin, Z. Bao, Y. Cui, Nat. Mater. 2022, 21, 445–454.
- 38J. Shi, T. Koketsu, Z. Zhu, M. Yang, L. Sui, J. Liu, M. Tang, Z. Deng, M. Liao, J. Xiang, Y. Shen, L. Qie, Y. Huang, P. Strasser, J. Ma, Nat. Mater. 2024, 23, 1686–1694.
- 39W. Zhang, Y. Lu, L. Wan, P. Zhou, Y. Xia, S. Yan, X. Chen, H. Zhou, H. Dong, K. Liu, Nat. Commun. 2022, 13, 2029.
- 40Y. Chen, Q. He, Y. Zhao, W. Zhou, P. Xiao, P. Gao, N. Tavajohi, J. Tu, B. Li, X. He, L. Xing, X. Fan, J. Liu, Nat. Commun. 2023, 14, 8326.
- 41S. Zhang, R. Li, T. Deng, Q. Ma, X. Hong, H. Zhang, R. Zhang, S. Ding, Y. Wu, H. Zhu, M. Li, H. Zhang, D. Lu, B. Ma, L. Lv, Y. Li, L. Chen, Y. Shen, R. Guo, X. Fan, Nat. Energy 2024, 9, 1285–1296.
- 42H. Shen, C. Song, F. Wang, G. Li, Y. Li, CCS Chemistry 2024, 6, 1300–1311.
- 43M. He, C. Li, H. Zhang, X. Chang, J. G. Chen, W. A. Goddard, M.-j. Cheng, B. Xu, Q. Lu, Nat. Commun. 2020, 11, 3844.
- 44L. Johnson, C. Li, Z. Liu, Y. Chen, S. A. Freunberger, P. C. Ashok, B. B. Praveen, K. Dholakia, J.-M. Tarascon, P. G. Bruce, Nat. Chem. 2014, 6, 1091–1099.
- 45Q. Wu, M. T. McDowell, Y. Qi, J. Am. Chem. Soc. 2023, 145, 2473–2484.
- 46D. Zhu, L. Sheng, Y. Ou, J. Wang, Y. Tang, K. Liu, X. He, H. Xu, ACS Appl. Mater. Interfaces 2025, 17, 13952–13959.