Ultrahigh-Voltage Lithium Metal Batteries Enabled by Single-Ion and Weakly-Solvating Nanometric Aggregates
Graphical Abstract
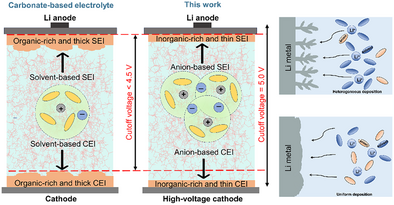
The table of contents (TOC) figure provides a visual overview of the key innovations and findings of this study on high-voltage lithium metal batteries (LMBs). The single-solvent electrolyte composed of 2.3 M lithium salt in tris(2,2,2-trifluoroethyl)phosphate (TFEP), which enables stable LMB operation at ultra-high voltages up to 5.0 V. The electrolyte forms large weakly-solvating nanometric aggregates (n-AGG), which contribute to enhanced oxidative stability and facilitate efficient redox reactions. Notably, the electrolyte exhibits exceptional cycling stability for over 1500 h, effectively suppressing dendrite formation and demonstrating strong compatibility with high-voltage cathodes, such as NCM811 and LiNi0.5Mn1.5O4 (LNMO). The improved thermodynamic and kinetic stability of the electrolyte significantly enhances the high-voltage tolerance and cycling performance of LMBs, offering promising prospects for the development of next-generation energy storage technologies.
Abstract
The urgent need for high energy density (> 400 Wh kg−1) has driven advancements in lithium metal batteries (LMBs) with high-voltage cathodes. However, degradation of traditional electrolytes restricts high cut-off voltage < 4.4 V, while low lithium transference numbers (tLi+) lead to polarization and early charge/discharge termination, which typically necessitate use of multiple solvents or salt-concentrated electrolytes to enable high-voltage chemistry. To address this challenge, we developed a single-solvent, single-salt electrolyte with tris(2,2,2-trifluoroethyl)phosphate (TFEP), achieving a high tLi+ of 0.78 and enabling ultra-high-voltage LMB operation up to 5.0 V. Large molecular sterics and electron density delocalization of TFEP enabled dominant presence of local aggregates (AGGs), which further populated to form large and ion-rich weakly-solvating nanometric aggregates (n-AGGs), changing redox properties and promoting the interfacial stabilities to a greater extent. As a result, we showed suppressed dendrite formation with stable cycling for over 1500 h, and full-cell operations paired with LiNi0.8Mn0.1Co0.1O2 (NCM811) at 4.7 V and with LiNi0.5Mn1.5O4 (LNMO) at 5.0 V. The tuning of bulk electrolyte properties from the scale of microscopic electronic structures to mesoscopic solvation structures has effectively enhanced thermodynamic and kinetic stabilities of the electrolyte, paving the way for LMBs with high-voltage tolerance.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.