Lewis-Base Electrolyte Additive Mediates Interfacial Chemistry for Stable Lithium Metal Batteries
Rong Fang
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Siyuan Ma
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
Both authors contributed equally to this work.
Search for more papers by this authorLian Ding
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Dr. Yu Gu
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorXin Dong
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Duan-Hui Si
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002 China
Search for more papers by this authorCorresponding Author
Prof. Jing-Hua Tian
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Xiu-Mei Lin
College of Chemistry, Chemical Engineering and Environment, Minnan Normal University, Zhangzhou, 363000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorProf. Bing-Wei Mao
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Jian-Feng Li
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
College of Chemistry, Chemical Engineering and Environment, Minnan Normal University, Zhangzhou, 363000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorRong Fang
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Siyuan Ma
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
Both authors contributed equally to this work.
Search for more papers by this authorLian Ding
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Dr. Yu Gu
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorXin Dong
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Duan-Hui Si
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002 China
Search for more papers by this authorCorresponding Author
Prof. Jing-Hua Tian
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Xiu-Mei Lin
College of Chemistry, Chemical Engineering and Environment, Minnan Normal University, Zhangzhou, 363000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
Search for more papers by this authorProf. Bing-Wei Mao
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Jian-Feng Li
College of Chemistry and Chemical Engineering, State Key Laboratory of Physical Chemistry of Solid Surfaces, the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Energy, Xiamen University, Xiamen, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, 361005 China
College of Chemistry, Chemical Engineering and Environment, Minnan Normal University, Zhangzhou, 363000 China
E-mail: [email protected]; [email protected]; [email protected]; [email protected]
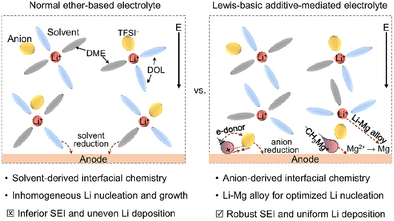
Search for more papers by this authorGraphical Abstract
A feasible ether-based electrolyte incorporating methylmagnesium chloride as a Lewis-basic additive is proposed to regulate interfacial chemistry. This electrolyte plays a crucial role in promoting a robust solid-electrolyte interphase formation and enhancing Li deposition, achieved through the bifunctional effects of anion-additive interactions. The designed electrolyte contributes to the superior cycling stability of Li metal anodes.
Abstract
Electrolytes play a crucial role in regulating interfacial chemistry, which is essential for the development of high-energy-density lithium metal batteries. Herein, we present an ether-based electrolyte system incorporating the simplest Grignard reagent, CH3MgCl, as an additive. This additive, endowed with Lewis-base characteristics, enhances the stability of the anode-electrolyte interface through bifunctional effects. During Li deposition, CH3Mg+ preferentially adsorbs onto the electrode surface, attracting more anions into the Helmholtz layer. Concurrently, CH3− creates an electron-rich environment, facilitating nucleophilic attacks on anions and promoting its reduction to form an inorganic-rich solid-electrolyte interphase (SEI). Additionally, Mg2+ undergoes electrodeposition prior to Li+, forming a Li-Mg alloy with subsequently deposited Li. This process lowers the nucleation barrier for Li deposition, resulting in improved deposition uniformity. Accordingly, the designed electrolyte demonstrates excellent cycling stability for Li anodes in both Li||Cu half-cells and full-cells paired with LiFePO4 cathodes. Notably, Li||LiFePO4 batteries using a thin-film Li anode pre-deposited on Cu retain ∼92.84% of their initial capacity after 300 cycles with an average Coulombic efficiency of ∼99.74%. These findings highlight the critical role of additives in engineering interfacial chemistry and provide a promising strategy for designing advanced electrolytes to improve the cycling performance of Li metal batteries.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202502048-sup-0001-SuppMat.docx6.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Kim, G. Park, S. J. Lee, S. Seo, K. Ryu, C. H. Kim, J. W. Choi, Adv. Mater. 2023, 35, 2206625.
- 2N. Jones, Nature 2024, 626, 248–251.
- 3Q. Li, H. Liu, F. Wu, L. Li, Y. Ye, R. Chen, Angew. Chem. Int. Ed. 2024, 63, e202404554.
- 4X.-B. Cheng, R. Zhang, C.-Z. Zhao, Q. Zhang, Chem. Rev. 2017, 117, 10403–10473.
- 5J.-G. Zhang, W. Xu, J. Xiao, X. Cao, J. Liu, Chem. Rev. 2020, 120, 13312–13348.
- 6C. Wei, Y. Zhang, Y. Tian, L. Tan, Y. An, Y. Qian, B. Xi, S. Xiong, J. Feng, Y. Qian, Energy Storage Mater. 2021, 38, 157–189.
- 7H. Yuan, X. Ding, T. Liu, J. Nai, Y. Wang, Y. Liu, C. Liu, X. Tao, Mater. Today 2022, 53, 173–196.
- 8 Chinese Society of Electrochemistry, J. Electrochem. 2024, 30, 2024121.
- 9R. Fang, Y.-X. Li, W.-W. Wang, Y. Gu, B.-W. Mao, Phys. Chem. Chem. Phys. 2024, 26, 23544–23560.
- 10S. Gu, Y. Zhang, M. Li, Q. Lin, G. Xu, N. Zhang, Angew. Chem. Int. Ed. 2025, 64, e202410020.
- 11W. Dachraoui, R. Pauer, C. Battaglia, R. Erni, ACS Nano 2023, 17, 20434–20444.
- 12X. Gao, Y.-N. Zhou, D. Han, J. Zhou, D. Zhou, W. Tang, J. B. Goodenough, Joule 2020, 4, 1864–1879.
- 13Y. Gu, H. Yan, W.-W. Wang, X.-G. Zhang, J. Yan, B.-W. Mao, Nano Lett. 2023, 23, 9872–9879.
- 14M. J. Zachman, Z. Tu, S. Choudhury, L. A. Archer, L. F. Kourkoutis, Nature 2018, 560, 345–349.
- 15B. Jagger, M. Pasta, Joule 2023, 7, 2228–2244.
- 16X. Sun, X. Zhang, Q. Ma, X. Guan, W. Wang, J. Luo, Angew. Chem. Int. Ed. 2020, 59, 6665–6674.
- 17K. N. Wood, M. Noked, N. P. Dasgupta, ACS Energy Lett. 2017, 2, 664–672.
- 18Y. Gu, E.-M. You, J.-D. Lin, J.-H. Wang, S.-H. Luo, R.-Y. Zhou, C.-J. Zhang, J.-L. Yao, H.-Y. Li, G. Li, W.-W. Wang, Y. Qiao, J.-W. Yan, D.-Y. Wu, G.-K. Liu, L. Zhang, J.-F. Li, R. Xu, Z.-Q. Tian, Y. Cui, B.-W. Mao, Nat. Commun. 2023, 14, 3536.
- 19H. Wang, Z. Yu, X. Kong, S. C. Kim, D. T. Boyle, J. Qin, Z. Bao, Y. Cui, Joule 2022, 6, 588–616.
- 20Y. S. Meng, V. Srinivasan, K. Xu, Science 2022, 378, 1065–1073.
- 21Q. Zhao, S. Stalin, L. A. Archer, Joule 2021, 5, 1119–1142.
- 22Y. Gu, W.-W. Wang, Y.-J. Li, Q.-H. Wu, S. Tang, J.-W. Yan, M.-S. Zheng, D.-Y. Wu, C.-H. Fan, W.-Q. Hu, Z.-B. Chen, Y. Fang, Q.-H. Zhang, Q.-F. Dong, B.-W. Mao, Nat. Commun. 2018, 9, 1339.
- 23A. R. Neale, R. Sharpe, S. R. Yeandel, C.-H. Yen, K. V. Luzyanin, P. Goddard, E. A. Petrucco, L. J. Hardwick, Adv. Funct. Mater. 2021, 31, 2010627.
- 24X. Zhang, P. Xu, J. Duan, X. Lin, J. Sun, W. Shi, H. Xu, W. Dou, Q. Zheng, R. Yuan, J. Wang, Y. Zhang, S. Yu, Z. Chen, M. Zheng, J.-F. Gohy, Q. Dong, A. Vlad, Nat. Commun. 2024, 15, 536.
- 25P. Xiao, X. Yun, Y. Chen, X. Guo, P. Gao, G. Zhou, C. Zheng, Chem. Soc. Rev. 2023, 52, 5255–5316.
- 26Z. Piao, R. Gao, Y. Liu, G. Zhou, H.-M. Cheng, Adv. Mater. 2023, 35, 2206009.
- 27Z. M. Hao-Ran Cheng, Y.-J. Guo, C.-S. Sun, Q. Li, J. Ming, J. Electrochem. 2022, 28, 2219012.
- 28H. Cheng, Q. Sun, L. Li, Y. Zou, Y. Wang, T. Cai, F. Zhao, G. Liu, Z. Ma, W. Wahyudi, Q. Li, J. Ming, ACS Energy Lett. 2022, 7, 490–513.
- 29Y. Chen, M. Li, Y. Liu, Y. Jie, W. Li, F. Huang, X. Li, Z. He, X. Ren, Y. Chen, X. Meng, T. Cheng, M. Gu, S. Jiao, R. Cao, Nat. Commun. 2023, 14, 2655.
- 30Y. Yamada, J. Wang, S. Ko, E. Watanabe, A. Yamada, Nat. Energy 2019, 4, 269–280.
- 31X.-T. Yang, C. Han, Y.-M. Xie, R. Fang, S. Zheng, J.-H. Tian, X.-M. Lin, H. Zhang, B.-W. Mao, Y. Gu, Y.-H. Wang, J.-F. Li, Small 2024, 20, 2311393.
- 32C. M. Efaw, Q. Wu, N. Gao, Y. Zhang, H. Zhu, K. Gering, M. F. Hurley, H. Xiong, E. Hu, X. Cao, W. Xu, J.-G. Zhang, E. J. Dufek, J. Xiao, X.-Q. Yang, J. Liu, Y. Qi, B. Li, Nat. Mater. 2023, 22, 1531–1539.
- 33X. Cao, H. Jia, W. Xu, J.-G. Zhang, J. Electrochem. Soc. 2021, 168, 010522.
- 34P. Thuy Duong, A. Bin Faheem, J. Kim, H. M. Oh, K.-K. Lee, Small 2022, 18, 2107492.
- 35Y.-X. Yao, X. Chen, C. Yan, X.-Q. Zhang, W.-L. Cai, J.-Q. Huang, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 4090–4097.
- 36T. Hu, J. Tian, F. Dai, X. Wang, R. Wen, S. Xu, J. Am. Chem. Soc. 2023, 145, 1327–1333.
- 37R. Guo, K.-H. Kim, B. M. Gallant, J. Electrochem. Soc. 2022, 169, 100523.
- 38S. Ye, L. Wang, F. Liu, P. Shi, H. Wang, X. Wu, Y. Yu, Adv. Energy Mater. 2020, 10, 2002647.
- 39Y. Gu, W.-W. Wang, J.-W. Yan, D.-Y. Wu, Q.-F. Dong, B.-W. Mao, Curr. Opin. Electrochem. 2021, 26, 100671.
- 40Y. Xu, H. Jia, P. Gao, D. E. Galvez-Aranda, S. P. Beltran, X. Cao, P. M. L. Le, J. Liu, M. H. Engelhard, S. Li, G. Ren, J. M. Seminario, P. B. Balbuena, J.-G. Zhang, W. Xu, C. Wang, Nat. Energy 2023, 8, 1345–1354.
- 41L. Liu, Z. Shadike, N. Wang, Y. Chen, X. Cai, E. Hu, J. Zhang, eScience 2024, 4, 100268.
- 42Y. Xiao, X. Wang, K. Yang, J. Wu, Y. Chao, C. Xi, M. Li, Q. Zhang, Z. Liu, L. Li, Y. Yu, C. Yang, Energy Storage Mater. 2023, 55, 773–781.
- 43J. Xu, V. Koverga, A. Phan, A. Min Li, N. Zhang, M. Baek, C. Jayawardana, B. L. Lucht, A. T. Ngo, C. Wang, Adv. Mater. 2023, 2306462.
- 44S. Kim, K.-Y. Cho, J. Kwon, K. Sim, D. Seok, H. Tak, J. Jo, K. Eom, Small 2023, 19, 2207222.
- 45S. Liu, X. Yu, Y. Yan, T. Zeng, X. Wang, G. Tian, C. Wang, S. Wang, Y. Zeng, C. Shu, Energy Storage Mater. 2023, 62, 102959.
- 46H. Zhuang, H. Xiao, T. Zhang, F. Zhang, P. Han, M. Xu, W. Dai, J. Jiao, L. Jiang, Q. Gao, Angew. Chem. Int. Ed. 2024, 63, e202407315.
- 47Y. Liu, X. Tao, Y. Wang, C. Jiang, C. Ma, O. Sheng, G. Lu, X. W. Lou, Science 2022, 375, 739–745.
- 48Q. Lin, D. Kundu, M. Skyllas-Kazacos, J. Lu, D. Zhao, K. Amine, L. Dai, D.-W. Wang, Adv. Mater. 2024, 36, 2406151.
- 49K. Ryu, K. Lee, J. Lim, M. J. Lee, K.-H. Kim, U. H. Lee, B. L. D. Rinkel, K. Kim, S. Kim, D. Kim, D. Shin, B. McCloskey, J. Kang, S. W. Lee, Energy Environ. Sci. 2024, 17, 7772–7781.
- 50J.-F. Ding, R. Xu, X.-X. Ma, Y. Xiao, Y.-X. Yao, C. Yan, J.-Q. Huang, Angew. Chem. Int. Ed. 2022, 61, e202115602.
- 51D. Aurbach, M. Moshkovich, A. Schechter, R. Turgeman, Electrochem. Solid-State Lett. 1999, 3, 31.
10.1149/1.1390949 Google Scholar
- 52J. Wang, L. Xie, W. Wu, Y. Liang, M. Cao, C. Gao, Y. Bo, J. Zhang, J. Zhang, Energy Environ. Sci. 2024, 17, 9100–9111.
- 53Z. Li, X. Huang, L. Kong, N. Qin, Z. Wang, L. Yin, Y. Li, Q. Gan, K. Liao, S. Gu, T. Zhang, H. Huang, L. Wang, G. Luo, X. Cheng, Z. Lu, Energy Storage Mater. 2022, 45, 40–47.
- 54Q.-K. Zhang, S.-Y. Sun, M.-Y. Zhou, L.-P. Hou, J.-L. Liang, S.-J. Yang, B.-Q. Li, X.-Q. Zhang, J.-Q. Huang, Angew. Chem. Int. Ed. 2023, 62, e202306889.
- 55W. Yu, Z. Yu, Y. Cui, Z. Bao, ACS Energy Lett. 2022, 7, 3270–3275.
- 56J. Jiang, X. Hu, S. Lu, C. Shen, S. Huang, X. Liu, Y. Jiang, J. Zhang, B. Zhao, Energy Storage Mater. 2023, 54, 885–894.
- 57Y.-Y. Wang, Y.-N. Wang, N. Yao, S.-Y. Sun, X.-Q. Ding, C.-X. Bi, Q.-K. Zhang, Z. Zheng, C.-B. Jin, B.-Q. Li, X.-Q. Zhang, J.-Q. Huang, J. Energy Chem. 2024, 95, 644–650.
- 58H. Li, C. Hong, R. Tao, X. Liu, J. Wang, J. Chen, S. Yao, J. Geng, G. Zheng, J. Liang, J. Energy Chem. 2025, 101, 416–428.
- 59B. Zhang, W. Zou, Z. Ju, S. Qi, J. Luo, C. J. Zhang, X. Tao, L. Du, ACS Nano 2023, 17, 22755–22765.
- 60H. Wang, J. Liu, G. Jiang, J. Huang, D. Wu, G. Yang, J. Ma, Adv. Energy Mater. 2024, 14, 2400067.
- 61B. Tong, J. Wang, Z. Liu, L. Ma, P. Wang, W. Feng, Z. Peng, Z. Zhou, J. Power Sources 2018, 400, 225–231.
- 62C. Zhang, Q. Lan, Y. Liu, J. Wu, H. Shao, H. Zhan, Y. Yang, Electrochim. Acta 2019, 306, 407–419.
- 63B. N. Olana, S. D. Lin, B.-J. Hwang, Electrochim. Acta 2022, 416, 140266.
- 64D. T. Boyle, X. Kong, A. Pei, P. E. Rudnicki, F. Shi, W. Huang, Z. Bao, J. Qin, Y. Cui, ACS Energy Lett. 2020, 5, 701–709.
- 65L. Pang, H. Li, X. Feng, Z. Zhao, C. Ouyang, Z. Peng, ACS Energy Lett. 2024, 9, 3587–3594.
- 66B. D. Adams, J. Zheng, X. Ren, W. Xu, J.-G. Zhang, Adv. Energy Mater. 2018, 8, 1702097.
- 67B. Dec, R. Bogdanowicz, K. Pyrchla, Photonics Lett. Pol. 2018, 10, 94.
- 68A. D. Becke, J. Chem. Phys. 1992, 96, 2155–2160.
- 69M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, et al., Wallingford, CT, 2016.
- 70T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 71J. Zhang, T. Lu, Phys. Chem. Chem. Phys. 2021, 23, 20323–20328.
- 72W. Humphrey, A. Dalke, K. Schulten, J. Mol. Graph. 1996, 14, 33–38.
- 73G. Kresse, J. Hafner, Phys. Rev. B 1993, 47, 558–561.
- 74G. Kresse, J. Hafner, Phys. Rev. B 1994, 49, 14251–14269.
- 75G. Kresse, J. Furthmüller, Phys. Rev. B 1996, 54, 11169–11186.
- 76J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 77P. E. Blöchl, Phys. Rev. B 1994, 50, 17953–17979.
- 78S. Grimme, WIREs Comput. Mol. Sci. 2011, 1, 211–228.