Electronically Asynchronous Transition State Tuned from Remote Site for Oxygen Atom Transfer by CuII–Nitrite Complexes
Jyoti Devi
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
Search for more papers by this authorCorresponding Author
Dr. Anannya Saha
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Suman K. Barman
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
E-mail: [email protected]; [email protected]
Search for more papers by this authorJyoti Devi
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
Search for more papers by this authorCorresponding Author
Dr. Anannya Saha
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Suman K. Barman
Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Knowledge City, Sector 81, Manauli PO 140306, SAS Nagar, Punjab, India
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
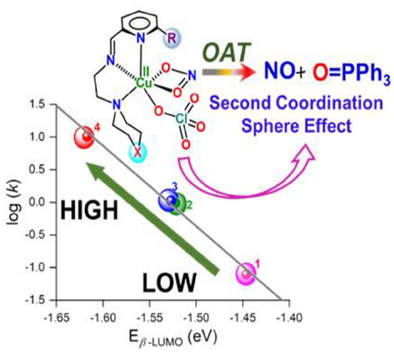
This work demonstrates the occurrence of electronically asynchronous transition state in oxygen atom transfer (OAT) reaction by CuII–NO2− complexes (1−4) controlled from remote site. OAT reactivity was found to be linearly correlated with β-LUMO and Cu(II)/(I) redox potentials, which were tuned from remote site substituents. Mechanistic study indicates asynchronous electron transfer mechanism where CuII/I reduction precedes nitrite reduction.
Abstract
Nitrite (NO2−) reduction to nitric oxide (NO) is of paramount interest in biology. In biology, Cu–nitrite reductase reduces NO2− to NO, while alternatively NO2− can be reduced to NO at copper center via oxygen atom transfer (OAT) to electron-rich substrate like PPh3. This work demonstrates systematic tuning of lowest unoccupied molecular orbital (LUMO) energy by remote site modification, which leads to systematic change in electrochemical property and OAT activity of CuII–NO2− involving electronically asynchronous transition state. For this purpose, we report here four CuII–NO2− complexes: [CuII(LCH2)(NO2)(ClO4)] (1), [CuII(LO)(NO2)(ClO4)] (2), [CuII(LCH2Me)(NO2)(ClO4)] (3), [CuII(LOMe)(NO2)(ClO4)] (4) with similar primary coordination spheres but different substituents at remote sites. In going from 1 to 4, by remote site substitution, there is systematic stabilization of LUMO energy, which correlates linearly with the increased OAT to PPh3 resulting in 130 times reactivity enhancement for 4 compared to 1. This kind of significant reactivity enhancement by tuning LUMO energy from remote site is very rare. Mechanistic study involving experimental and computational study reveals asynchronous mechanism that was hitherto not reported for any OAT. The observed increase in OAT reactivity from 1 to 4 is attributed to an increase in the extent of asynchronicity in corresponding transition states, which was controlled from remote site modification.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
All experimental data, detailed experimental as well as computational methods and optimised coordinates are available in the Supporting information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202501338-sup-0001-SuppMat.pdf3.5 MB | Supporting Information |
| anie202501338-sup-0002-SuppMat.cif6.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. B. Maia, J. J. G. Moura, Chem. Rev. 2010, 110, 6474–6502.
10.1021/cr100246c Google Scholar
- 2M. T. Gladwin, A. N. Schechter, D. B. Kim-Shapiro, R. P. Patel, N. Hogg, S. Shiva, R. O. Cannon, M. Kelm, D. A. Wink, M. G. Espey, E. H. Oldfield, R. M. Pluta, B. A. Freeman, J. R. Lancaster Jr., M. Feelisch, J. O. Lundberg, Nat. Chem. Biol. 2005, 1, 308–314.
- 3S. A. Omar, A. J. Webb, J. Mol. Cell. Cardiol. 2014, 73, 57–69.
- 4A. J. Timmons, M. D. Symes, Chem. Soc. Rev. 2015, 44, 6708–6722.
- 5 Nitric Oxide: Biology and Pathobiology, 3rd ed. (Eds: L. J. Ignarro, B. Freeman), Elsevier/Academic Press, Amsterdam, The Netherlands 2010.
- 6B. P. Luchsinger, E. N. Rich, Y. Yan, E. M. Williams, J. S. Stamler, D. J. Singel, J. Inorg. Biochem. 2005, 99, 912–921.
- 7M. T. Gladwin, Nat. Chem. Biol. 2005, 1, 245–246.
- 8J. O. Lundberg, M. T. Gladwin, E. Weitzberg, Nat. Rev. Drug Discov. 2015, 14, 623–641.
- 9S. Basu, N. A. Azarova, M. D. Font, S. B. King, N. Hogg, M. T. Gladwin, S. Shiva, D. B. Kim-Shapiro, J. Biol. Chem. 2008, 283, 32590–32597.
- 10K. Cosby, K. S. Partovi, J. H. Crawford, R. P. Patel, C. D. Reiter, S. Martyr, B. K. Yang, M. A. Waclawiw, G. Zalos, X. Xu, K. T. Huang, H. Shields, D. B. Kim-Shapiro, A. N. Schechter, R. O. Cannon, M. T. Gladwin, Nat. Med. 2003, 9, 1498–1505.
- 11S. Shiva, Z. Huang, R. Grubina, J. Sun, L. A. Ringwood, P. H. MacArthur, X. Xu, E. Murphy, V. M. Darley-Usmar, M. T. Gladwin, Circ. Res. 2007, 100, 654–661.
- 12L. B. Maia, J. J. G. Moura, Chem. Rev. 2014, 114, 5273–5357.
- 13J. A. Camargo, Á. Alonso, Environ. Int. 2006, 32, 831–849.
- 14J. A. Camargo, A. Alonso, A. Salamanca, Chemosphere 2005, 58, 1255–1267.
- 15B. A. Averill, Chem. Rev. 1996, 96, 2951–2964.
- 16A. C. Merkle, N. Lehnert, Dalton Trans. 2012, 41, 3355–3368.
- 17W.-M. Ching, C.-H. Chuang, C.-W. Wu, C.-H. Peng, C.-H. Hung, J. Am. Chem. Soc. 2009, 131, 7952–7953.
- 18C.-C. Tsou, W.-L. Yang, W.-F. Liaw, J. Am. Chem. Soc. 2013, 135, 18758–18761.
- 19E. M. Matson, Y. J. Park, A. R. Fout, J. Am. Chem. Soc. 2014, 136, 17398–17401.
- 20Y. J. Park, M. N. Peñas-Defrutos, M. J. Drummond, Z. Gordon, O. R. Kelly, I. J. Garvey, K. L. Gullett, M. García-Melchor, A. R. Fout, Inorg. Chem. 2022, 61, 8182–8192.
- 21B. C. Sanders, S. M. Hassan, T. C. Harrop, J. Am. Chem. Soc. 2014, 136, 10230–10233.
- 22J. A. Halfen, S. Mahapatra, E. C. Wilkinson, A. J. Gengenbach, V. G. Young, L. Que, W. B. Tolman, J. Am. Chem. Soc. 1996, 118, 763–776.
- 23L. Casella, O. Carugo, M. Gullotti, S. Doldi, M. Frassoni, Inorg. Chem. 1996, 35, 1101–1113.
- 24N. Lehnert, U. Cornelissen, F. Neese, T. Ono, Y. Noguchi, K. Okamoto, K. Fujisawa, Inorg. Chem. 2007, 46, 3916–3933.
- 25C.-S. Chen, W.-Y. Yeh, Chem. Commun. 2010, 46, 3098–3100.
- 26M. Kujime, C. Izumi, M. Tomura, M. Hada, H. Fujii, J. Am. Chem. Soc. 2008, 130, 6088–6098.
- 27W. J. Chuang, I. J. Lin, H. Y. Chen, Y. L. Chang, S. C. N. Hsu, Inorg. Chem. 2010, 49, 5377–5384.
- 28M. Kumar, N. A. Dixon, A. C. Merkle, M. Zeller, N. Lehnert, E. T. Papish, Inorg. Chem. 2012, 51, 7004–7006.
- 29S. C. N. Hsu, Y. Chang, W. Chuang, H. Chen, I. Lin, M. Y. Chiang, C. Kao, H. Chen, Inorg. Chem. 2012, 51, 9297–9308.
- 30R. C. Maji, S. K. Barman, S. Roy, S. K. Chatterjee, F. L. Bowles, M. M. Olmstead, A. K. Patra, Inorg. Chem. 2013, 52, 11084–11095.
- 31R. C. Maji, S. Mishra, A. Bhandari, R. Singh, M. M. Olmstead, A. K. Patra, Inorg. Chem. 2018, 57, 1550–1561.
- 32Z. Sakhaei, S. Kundu, J. M. Donnelly, J. A. Bertke, W. Y. Kim, T. H. Warren, Chem. Commun. 2017, 53, 549–552.
- 33S. Kundu, W. Y. Kim, J. A. Bertke, T. H. Warren, J. Am. Chem. Soc. 2017, 139, 1045–1048.
- 34M. Stauffer, Z. Sakhaei, C. Greene, P. Ghosh, J. A. Bertke, T. H. Warren, Inorg. Chem. 2021, 60, 15968–15974.
- 35S. Maria, T. Chattopadhyay, S. Ananya, S. Kundu, Inorg. Chim. Acta 2020, 506, 119515.
- 36K. Chand, Y.-C. Chu, T.-W. Wang, C.-L. Kao, Y.-F. Lin, M.-L. Tsai, S. C. N. Hsu, Dalton Trans. 2022, 51, 3485–3496.
- 37S. L. Rose, S. V. Antonyuk, D. Sasaki, K. Yamashita, K. Hirata, G. Ueno, H. Ago, R. R. Eady, T. Tosha, M. Yamamoto, S. S. Hasnain, Sci. Adv. 2021, 7, eabd8523.
- 38Y. Fukuda, K. M. Tse, T. Nakane, T. Nakatsu, M. Suzuki, M. Sugahara, S. Inoue, T. Masuda, F. Yumoto, N. Matsugaki, E. Nango, K. Tono, Y. Joti, T. Kameshima, C. Song, T. Hatsui, M. Yabashi, O. Nureki, M. E. P. Murphy, T. Inoue, S. Iwata, E. Mizohata, Proc. Natl. Acad. Sci. USA 2016, 113, 2928–2933.
- 39G. Cioncoloni, I. Roger, P. S. Wheatley, C. Wilson, R. E. Morris, S. Sproules, M. D. Symes, ACS Catal. 2018, 8, 5070–5084.
- 40C. M. Moore, N. K. Szymczak, Chem. Sci. 2015, 6, 3373–3377.
- 41A. P. Hunt, A. E. Batka, M. Hosseinzadeh, J. D. Gregory, H. K. Haque, H. Ren, M. E. Meyerhoff, N. Lehnert, ACS Catal. 2019, 9, 7746–7758.
- 42P. H. van Langevelde, S. Engbers, F. Buda, D. G. H. Hetterscheid, ACS Catal. 2023, 13, 10094–10103.
- 43C. E. Elwell, N. L. Gagnon, B. D. Neisen, D. Dhar, A. D. Spaeth, G. M. Yee, W. B. Tolman, Chem. Rev. 2017, 117, 2059–2107.
- 44D. Maiti, H. R. Lucas, A. A. N. Sarjeant, K. D. Karlin, J. Am. Chem. Soc. 2007, 129, 6998–6999.
- 45N. R. M. de Kler, J. Roithová, Chem. Commun. 2020, 56, 12721–12724.
- 46G. P. Van Trieste, III, J. H. Reibenspies, Y.-S. Chen, D. Sengupta, R. R. Thompson, D. C. Powers, Chem. Commun. 2022, 58, 12608–12611.
- 47X. Huang, J. T. Groves, Chem. Rev. 2018, 118, 2491–2553.
- 48V. A. Larson, B. Battistella, K. Ray, N. Lehnhert, W. Nam, Nat. Rev. Chem. 2020, 4, 404–419.
- 49W. Nam, Y.-M. Lee, S. Fukuzumi, Acc. Chem. Res. 2014, 47, 1146–1154.
- 50Y.-M. Lee, M. Yoo, H. Yoon, X.-X. Li, W. Nam, S. Fukuzumi, Chem. Commun. 2017, 53, 9352–9355.
- 51Y. Li, R. Singh, A. Sinha, G. C. Lisensky, M. Haukka, J. Nilsson, S. Yiga, S. Demeshko, S. J. Gross, S. Dechert, A. Gonzalez, G. Farias, O. F. Wendt, F. Meyer, E. Nordlander, Inorg. Chem. 2023, 62, 18338–18356.
- 52P. Singh, Y. Lee, J. R. Mayfield, R. Singh, M. C. Denler, S. D. Jones, V. W. Day, E. Nordlander, T. A. Jackson, Inorg. Chem. 2023, 62, 18357–18374.
- 53C. Khin, J. Heinecke, P. C. Ford, J. Am. Chem. Soc. 2008, 130, 13830–13831.
- 54J. Heinecke, P. C. Ford, J. Am. Chem. Soc. 2010, 132, 9240–9243.
- 55J. L. Heinecke, C. Khin, J. C. M. Pereira, S. A. Suárez, A. V. Iretskii, F. Doctorovich, P. C. Ford, J. Am. Chem. Soc. 2013, 135, 4007–4017.
- 56T. S. Kurtikyan, A. A. Hovhannisyan, A. V. Iretskii, P. C. Ford, Inorg. Chem. 2009, 48, 11236–11241.
- 57J. Goodwin, T. Kurtikyan, J. Standard, R. Walsh, B. Zheng, D. Parmley, J. Howard, S. Green, A. Mardyukov, D. E. Przybyla, Inorg. Chem. 2005, 44, 2215–2223.
- 58J. Goodwin, R. Bailey, W. Pennington, R. Rasberry, T. Green, S. Shasho, M. Yongsavanh, V. Echevarria, J. Tiedeken, C. Brown, G. Fromm, S. Lyerly, N. Watson, A. Long, N. De Nitto, Inorg. Chem. 2001, 40, 4217–4225.
- 59J. Paudel, A. Pokhrel, M. L. Kirk, F. Li, Inorg. Chem. 2019, 58, 2054–2068.
- 60K. Peariso, R. L. McNaughton, M. L. Kirk, J. Am. Chem. Soc. 2002, 124, 9006–9007.
- 61H. Nagao, N. Komeda, M. Mukaida, M. Suzuki, K. Tanaka, Inorg. Chem. 1996, 35, 6809–6815.
- 62M. D. Lim, I. M. Lorkovic, P. C. Ford, Inorg. Chem. 2002, 41, 1026–1028.
- 63P. Mondal, I. Ishigami, S. Yeh, G. B. Wijeratne, Angew. Chem. Int. Ed. 2022, 61, e202211521.
- 64M. T. Kieber-Emmons, J. Annaraj, M. S. Seo, K. M. Van Heuvelen, T. Tosha, T. Kitagawa, T. C. Brunold, W. Nam, C. G. Riordan, J. Am. Chem. Soc. 2006, 128, 14230–14231.
- 65J. Cho, J. Woo, W. Nam, J. Am. Chem. Soc. 2012, 134, 11112–11115.
- 66S. Fukuzumi, K. Shimoosako, T. Suenobu, Y. Watanabe, J. Am. Chem. Soc. 2003, 125, 9074–9082.
- 67F. Avenier, C. Herrero, W. Leibl, A. Desbois, R. Guillot, J.-P. Mahy, A. Aukauloo, Angew. Chem. Int. Ed. 2013, 52, 3634–3637.
- 68H. M. Neu, T. Yang, R. A. Bagila, T. H. Yosca, M. T. Green, M. G. Quesne, S. P. de Visser, D. P. Goldberg, J. Am. Chem. Soc. 2014, 136, 13845–13852.
- 69J. England, J. O. Bigelow, K. M. Van Heuvelen, E. R. Farquhar, M. Martinho, K. K. Meier, J. R. Frisch, E. Münck, L. Que, Chem. Sci. 2014, 5, 1204–1215.
- 70C. V. Sastri, J. Lee, K. Oh, Y. J. Lee, J. Lee, T. A. Jackson, K. Ray, H. Hirao, W. Shin, J. A. Halfen, J. Kim, L. Que Jr., S. Shaik, W. Nam, Proc. Natl. Acad. Sci. USA 2007, 104, 19181–19186.
- 71A. A. Opalade, J. D. Parham, V. W. Day, T. A. Jackson, Chem. Sci. 2021, 12, 12564–12575.
- 72G. Gupta, M. Bera, S. Paul, S. Paria, Inorg. Chem. 2021, 60, 18006–18016.
- 73K. Keshari, A. Santra, L. Velasco, M. Sauvan, S. Kaur, A. D. Ugale, S. Munshi, J. F. Marco, D. Munshiram, S. Paria, JACS Au 2024, 4, 1142–1154.
- 74S. Fukuzumi, K. Ohkubo, Y.-M. Lee, W. Nam, Chem. Eur. J. 2015, 21, 17548–17559.
- 75J. Park, Y. Morimoto, Y.-M. Lee, W. Nam, S. Fukuzumi, J. Am. Chem. Soc. 2011, 133, 5236–5239.
- 76J. Park, Y. Morimoto, Y.-M. Lee, W. Nam, S. Fukuzumi, J. Am. Chem. Soc. 2012, 134, 3903–3911.
- 77J. Park, Y.-M. Lee, W. Nam, S. Fukuzumi, J. Am. Chem. Soc. 2013, 135, 5052–5061.
- 78J. Park, Y. Morimoto, Y.-M. Lee, W. Nam, S. Fukuzumi, Inorg. Chem. 2014, 53, 3618–3628.
- 79J. Chen, H. Yoon, Y.-M. Lee, M. S. Seo, R. Sarangi, S. Fukuzumi, W. Nam, Chem. Sci. 2015, 6, 3624–3632.
- 80S. Fukuzumi, Coord. Chem. Rev. 2013, 257, 1564–1575.
- 81D. Bím, M. Maldonado-Domínguez, L. Rulíšek, M. Srnec, Proc. Natl. Acad. Sci. USA 2018, 115, E10287–E10294.
- 82M. K. Goetz, J. S. Anderson, J. Am. Chem. Soc. 2019, 141, 4051–4062.
- 83S. K. Barman, M.-Y. Yang, T. H. Parsell, M. T. Green, A. S. Borovik, Proc. Natl. Acad. Sci. USA 2021, 118, e2108648118.
- 84M. Molla, A. Saha, S. K. Barman, S. Mandal, Chem. Eur. J. 2024, 30, e202401163.
- 85R. Sun, S. Ruccolo, D. L. Nascimento, Y. Qin, N. Hibbert, D. G. Nocera, Chem. Sci. 2023, 14, 13776–13782.
- 86N. P. van Leest, M. A. Tepaske, B. Venderbosch, J.-P. H. Oudsen, M. Tromp, J. I. van der Vlugt, B. de Bruin, ACS Catal. 2020, 10, 7449–7463.
- 87X.-K. Jiang, Acc. Chem. Res. 1997, 30, 283–289.