Direct Conversion of Aromatic Lactones into Bioisosteres by Carbonyl-to-Boranol Exchange
Yu Zhang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorCorresponding Author
Hong Lu
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorJie Chang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorPeng-Fei Xu
State Key Laboratory of Applied Organic Chemistry College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 China
Search for more papers by this authorHang Li
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorYuan Jin
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorCorresponding Author
Hao Wei
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorYu Zhang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorCorresponding Author
Hong Lu
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorJie Chang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorPeng-Fei Xu
State Key Laboratory of Applied Organic Chemistry College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 China
Search for more papers by this authorHang Li
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorYuan Jin
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
Search for more papers by this authorCorresponding Author
Hao Wei
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
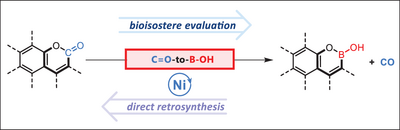
A nickel-catalyzed group-exchange strategy has been developed for the direct conversion of aromatic lactones into cyclic hemiboronic acid bioisosteres. Scope evaluation and product derivatization experiments demonstrate broad functional-group compatibility and the synthetic value of this strategy. Furthermore, the application of this methodology to the rapid modification of lactone cores in bioactive molecules underscores its practical utility.
Abstract
Bioisosteric replacement is an important strategy in drug discovery and is commonly practiced in medicinal chemistry; however, the incorporation of bioisosteres typically requires laborious multistep de novo synthesis. The direct conversion of a functional group into its corresponding bioisostere is of particular significance in evaluating structure-property relationships. Herein, we report a functional-group-exchange strategy that enables the direct conversion of aromatic lactones, a prevalent motif in bioactive molecules, into their corresponding cyclic hemiboronic acid bioisosteres. Scope evaluation and product derivatization experiments demonstrate the synthetic value and broad functional-group compatibility of this strategy, while the application of this methodology to the rapid remodeling of chromenone cores in bioactive molecules highlights its utility.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202500921-sup-0001-SuppMat.pdf12.7 MB | Supporting Information |
| anie202500921-sup-0002-SuppMat.cif286.5 KB | Supporting Information |
| anie202500921-sup-0003-SuppMat.cif711.1 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. A. Meanwell, J. Med. Chem. 2011, 54, 2529–2591.
- 2N. A. Meanwell, J. Agric. Food Chem. 2023, 71, 18087–18122.
- 3Y. Hamada, Y. Kiso, Expert Opin. Drug Discovery 2012, 7, 903–922.
- 4S. Kumari, A. V. Carmona, A. K. Tiwari, P. C. Trippier, J. Med. Chem. 2020, 63, 12290–12358.
- 5M. A. M. Subbaiah, N. A. Meanwell, J. Med. Chem. 2021, 64, 14046–14128.
- 6S. J. Baker, C. Z. Ding, T. Akama, Y. K. Zhang, V. Hernandez, Y. Xia, Future Med. Chem. 2009, 1, 1275–1288.
- 7A. Adamczyk-Woźniak, K. M. Borys, A. Sporzynski, Chem. Rev. 2015, 115, 5224–5247.
- 8H. S. Ban, H. Nakamura, Chem. Rec. 2015, 15, 616–635.
- 9M. Z. H. Kazmi, O. M. Schneider, D. G. Hall, J. Med. Chem. 2023, 66, 13768–13787.
- 10M. Z. H. Kazmi, J. P. G. Rygus, H. T. Ang, M. Paladino, M. A. Johnson, M. J. Ferguson, D. G. Hall, J. Am. Chem. Soc. 2021, 143, 10143–10156.
- 11J. Woo, C. Stein, A. H. Christian, M. D. Levin, Nature 2023, 623, 77–82.
- 12T. J. Pearson, R. Shimazumi, J. L. Driscoll, B. D. Dherange, D.-I. Park, M. D. Levin, Science 2023, 381, 1474–1479.
- 13S. C. Patel, N. Z. Burns, J. Am. Chem. Soc. 2022, 144, 17797–17802.
- 14H. Lu, Y. Zhang, X.-H. Wang, R. Zhang, P.-F. Xu, H. Wei, Nat. Commun. 2024, 15, 3772.
- 15H. Lyu, I. Kevlishvili, X. Yu, P. Liu, G. Dong, Science 2021, 372, 175–182.
- 16Z. Wu, X. Xu, J. Wang, G. Dong, Science 2021, 374, 734–740.
- 17E. Mao, C. N. P. Kullmer, H. A. Sakai, D. W. C. MacMillan, J. Am. Chem. Soc. 2024, 146, 5067–5073.
- 18J. Woo, A. H. Christian, S. A. Burgess, Y. Jiang, U. F. Mansoor, M. D. Levin, Science 2022, 376, 527–532.
- 19J. B. Roque, Y. Kuroda, L. T. Göttemann, R. Sarpong, Nature 2018, 564, 244–248.
- 20M. C. L. Jurczyk, D. Adpressa, S. F. Kim, Y.-H. Lam, C. S. Yeung, R. Sarpong, Science 2021, 373, 1004–1012.
- 21H. Zhong, D. T. Egger, V. C. M. Gasser, P. Finkelstein, L. Keim, M. Z. Seidel, N. Trapp, B. Morandi, Nat. Commun. 2023, 14, 5273.
- 22Q. Cheng, D. Bhattacharya, M. Haring, H. Cao, C. Mück-Lichtenfeld, A. Studer, Nat. Chem. 2024, 16, 741–748.
- 23D. Kim, J. You, D. H. Lee, H. Hong, D. Kim, Y. Park, Science 2024, 386, 99–105.
- 24Q. Peng, M. U. Hwang, Á. Rentería-Gómez, P. Mukherjee, R. M. Young, Y. Qiu, M. R. Wasielewski, O. Gutierrez, K. A. Scheidt, Science 2024, 385, 1471–1477.
- 25Z. A. Tolchin, J. M. Smith, J. Am. Chem. Soc. 2024, 146, 2939–2943.
- 26B. J. H. Uhlenbruck, C. M. Josephitis, L. de Lescure, R. S. Paton, S. McNally, Nature 2024, 631, 87–93.
- 27G. L. Bartholomew, S. L. Kraus, L. J. Karas, F. Carpaneto, R. Bennett, M. S. Sigman, C. S. Yeung, R. Sarpong, J. Am. Chem. Soc. 2024, 146, 2950–2958.
- 28J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong, M. D. Levin, Nat. Synth. 2022, 1, 352–364.
- 29H. Lu, T.-Y. Yu, P.-F. Xu, H. Wei, Chem. Rev. 2021, 121, 365–411.
- 30R. Takise, K. Muto, J. Yamaguchi, Chem. Soc. Rev. 2017, 46, 5864–5888.
- 31N. Lalloo, C. E. Brigham, M. S. Sanford, Acc. Chem. Res. 2022, 55, 3430–3444.
- 32X.-T. Min, D.-W. Ji, Y.-Q. Guan, S.-Y. Guo, Y.-C. Hu, B. Wan, Q.-A. Chen, Angew. Chem. Int. Ed. 2021, 60, 1583–1587.
- 33Z. Huang, M. E. Akana, K. M. Sanders, D. J. Weix, Science 2024, 385, 1331–1337.
- 34J. Wang, L. E. Ehehalt, Z. Huang, O. M. Beleh, I. A. Guzei, D. J. Weix, J. Am. Chem. Soc. 2023, 145, 9951–9958.
- 35R. Takise, R. Isshiki, K. Muto, K. Itami, J. Yamaguchi, J. Am. Chem. Soc. 2017, 139, 3340–3343.
- 36K. Muto, J. Yamaguchi, D. G. Musaev, K. Itami, Nat. Commun. 2015, 6, 7508.
- 37L. Guo, A. Chatupheeraphat, M. Rueping, Angew. Chem. Int. Ed. 2016, 55, 11810–11813.
- 38L. Guo, M. Rueping, Chem.-Eur. J. 2016, 22, 16787–16790.
- 39X. Pu, J. Hu, Y. Zhao, Z. Shi, ACS Catal. 2016, 6, 6692–6698.
- 40H. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, 15315–15318.
- 41Q. H. Luu, J. Li, Chem. Sci. 2022, 13, 1095–1100.
- 42Q. J. Zhou, K. Worm, R. E. Dolle, J. Org. Chem. 2004, 69, 5147–5149.
- 43N. I. Saper, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 17667–17676.
- 44C. Liu, M. Szostak, Org. Biomol. Chem. 2018, 16, 7998–8010.
- 45J. Liu, X. Jia, X. Chen, H. Sun, Y. Li, S. Kramer, Z. Lian, J. Org. Chem. 2020, 85, 5702–5711.
- 46F. Takahashi, K. Nogi, H. Yorimitsu, Org. Lett. 2018, 20, 6601–6605.
- 47Under deposition numbers CCDC 2376610 (1) and 2376611 (29). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
- 48Y. Sumida, R. Harada, T. Kato-Sumida, K. Johmoto; H. Uekusa, T. Hosoya, Org. Lett. 2014, 16, 6240–6243.
- 49G. P. Goryunov, M. I. Sharikov, A. N. Iashin, J. A. M. Canich, S. J. Mattler, J. R. Hagadorn, D. V. Uborsky, A. Z. Voskoboynikov, ACS Catal. 2021, 11, 8079–8086.
- 50Y. Zhao, L. Song, J. Chang, B. Liu, Org. Lett. 2024, 26, 1056–1061.
- 51T.-Z. Li, C.-A. Geng, J.-J. Chen, Tetrahedron Lett. 2019, 60, 151059.