On-Surface Synthesis of Nanographenes Through Domino Cyclization Reactions
Shijie Sun
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Both authors contributed equally to this work.
Search for more papers by this authorQingyan Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Both authors contributed equally to this work.
Search for more papers by this authorTakatsugu Onishi
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Quantum Materials Science Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorGoudappagouda
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorHangjing Zhou
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorProf. Lei Gao
Faculty of Science, Kunming University of Science and Technology, Kunming, Yunnan, 650500 China
Search for more papers by this authorProf. Yoshinori Okada
Quantum Materials Science Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorCorresponding Author
Prof. Jianchen Lu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Akimitsu Narita
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jinming Cai
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Southwest United Graduate School, Kunming, 650093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorShijie Sun
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Both authors contributed equally to this work.
Search for more papers by this authorQingyan Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Both authors contributed equally to this work.
Search for more papers by this authorTakatsugu Onishi
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Quantum Materials Science Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorGoudappagouda
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorHangjing Zhou
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorProf. Lei Gao
Faculty of Science, Kunming University of Science and Technology, Kunming, Yunnan, 650500 China
Search for more papers by this authorProf. Yoshinori Okada
Quantum Materials Science Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
Search for more papers by this authorCorresponding Author
Prof. Jianchen Lu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Akimitsu Narita
Organic and Carbon Nanomaterials Unit, Okinawa Institute of Science and Technology Graduate University, Kunigami-gun, Okinawa, 904-0495 Japan
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jinming Cai
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Southwest United Graduate School, Kunming, 650093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
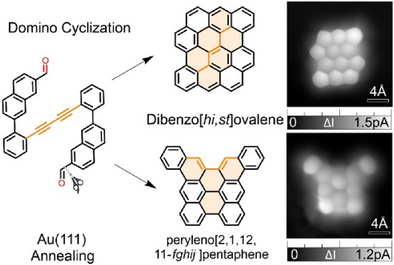
On-surface synthesis enables nanographene fabrication but with limited reactions. Here, bis{2-(7-formylnaphthalen-2-yl)phenyl}diacetylene undergoes domino cyclization via cycloisomerization, cyclodehydrogenation, and formyl cyclization, yielding dibenzo[hi,st]ovalene and peryleno[2,1,12,11-fghij]pentaphene. STM, STS, and DFT analyses reveal their structures and properties, advancing carbon nanostructure synthesis.
Abstract
On-surface synthesis has emerged as a powerful method to synthesize nanographenes that are difficult to obtain through the solution chemistry, but the number of available reactions is still highly limited. In this study, we demonstrate an unprecedented on-surface domino cyclization of bis{2-(7-formylnaphthalen-2-yl)phenyl}diacetylene, leading to dibenzo[hi,st]ovalene and peryleno[2,1,12,11-fghij]pentaphene through a sequence of 1) cycloisomerization of diaryldiacetylene moieties, 2) oxidative cyclodehydrogenation, and 3) reductive cyclization of formyl groups. The structures of these nanographenes and other cyclized products were unambiguously elucidated by using scanning tunneling microscopy directly on Au(111). Moreover, their electronic properties were investigated by scanning tunneling spectroscopy combined with density functional theory calculations. Our findings offer new insights into the on-surface cyclization reactions, providing an effective strategy for synthesizing a wider variety of carbon nanostructures.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202425167-sup-0001-SuppMat.docx7.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Liu, X. Feng, Angew. Chem. Int. Ed. 2020, 59, 23386–23401.
- 2Q. Fan, D. Martin-Jimenez, S. Werner, D. Ebeling, T. Koehler, T. Vollgraff, J. R. Sundermeyer, W. Hieringer, A. Schirmeisen, J. M. Gottfried, J. Am. Chem. Soc. 2020, 142, 894–899.
- 3S. Mishra, K. Xu, K. Eimre, H. Komber, J. Ma, C. A. Pignedoli, R. Fasel, X. Feng, P. Ruffieux, Nanoscale 2021, 13, 1624–1628.
- 4T. G. Lohr, J. I. Urgel, K. Eimre, J. Liu, M. Di Giovannantonio, S. Mishra, R. Berger, P. Ruffieux, C. A. Pignedoli, R. Fasel, J. Am. Chem. Soc. 2020, 142, 13565–13572.
- 5S. Mishra, X. Yao, Q. Chen, K. Eimre, O. Gröning, R. Ortiz, M. Di Giovannantonio, J. C. Sancho-García, J. Fernández-Rossier, C. A. Pignedoli, Nat. Chem. 2021, 13, 581–586.
- 6I. C.-Y. Hou, Q. Sun, K. Eimre, M. Di Giovannantonio, J. I. Urgel, P. Ruffieux, A. Narita, R. Fasel, K. Müllen, J. Am. Chem. Soc. 2020, 142, 10291–10296.
- 7K. Xu, J. I. Urgel, K. Eimre, M. Di Giovannantonio, A. Keerthi, H. Komber, S. Wang, A. Narita, R. Berger, P. Ruffieux, J. Am. Chem. Soc. 2019, 141, 7726–7730.
- 8H. Yang, Y. Cao, Y. Gao, Y. Fu, L. Huang, J. Liu, X. Feng, S. Du, H.-J. Gao, Chin. Phys. B 2021, 30, 056802.
- 9M. Di Giovannantonio, A. Keerthi, J. I. Urgel, M. Baumgarten, X. Feng, P. Ruffieux, A. Narita, R. Fasel, K. Müllen, J. Am. Chem. Soc. 2020, 142, 1721–1725.
- 10Y. Zheng, C. Li, C. Xu, D. Beyer, X. Yue, Y. Zhao, G. Wang, D. Guan, Y. Li, H. Zheng, Nat. Commun. 2020, 11, 6076.
- 11S. Wang, Q. Sun, O. Gröning, R. Widmer, C. A. Pignedoli, L. Cai, X. Yu, B. Yuan, C. Li, H. Ju, Nat. Chem. 2019, 11, 924–930.
- 12S. Sun, Y. Guan, Z. Hao, Z. Ruan, H. Zhang, J. Lu, L. Gao, X. Zuo, J. Cai, Nano Res. 2022, 15, 653–658.
- 13X. Xu, M. Di Giovannantonio, J. I. Urgel, C. A. Pignedoli, P. Ruffieux, K. Müllen, R. Fasel, A. Narita, Nano Res. 2021, 14, 4754–4759.
- 14Y. Zhang, J. Lu, Y. Li, B. Li, Z. Ruan, H. Zhang, Z. Hao, S. Sun, W. Xiong, L. Gao, L. Chen, J. Cai, Angew. Chem. Int. Ed. 2022, 61, e202204736.
- 15X. Xu, A. Kinikar, M. D. Giovannantonio, P. Ruffieux, K. Müllen, R. Fasel, A. Narita, Bull. Chem. Soc. Jpn. 2021, 94, 997–999.
- 16R. Zuzak, J. Castro-Esteban, M. Engelund, D. Pérez, D. Peña, S. Godlewski, ACS Nano 2023, 17, 2580–2587.
- 17J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Mullen, R. Fasel, Nature 2010, 466, 470–473.
- 18L. Talirz, P. Ruffieux, R. Fasel, Adv. Mater. 2016, 28, 6222–6231.
- 19G. M. Paternò, Q. C. Goudappagouda, G. Lanzani, F. Scotognella, A. Narita, Adv. Opt. Mater. 2021, 9, 2100508.
- 20J. Liu, S. Mishra, C. A. Pignedoli, D. Passerone, J. I. Urgel, A. Fabrizio, T. G. Lohr, J. Ma, H. Komber, M. Baumgarten, C. Corminboeuf, R. Berger, P. Ruffieux, K. Müllen, R. Fasel, X. Feng, J. Am. Chem. Soc. 2019, 141, 12011–12020.
- 21J. Lawrence, M. S. Mohammed, D. Rey, F. Aguilar-Galindo, A. Berdonces-Layunta, D. Peña, D. G. de Oteyza, ACS Nano 2021, 15, 4937–4946.
- 22W. Yang, R. Bam, V. J. Catalano, W. A. Chalifoux, Angew. Chem. Int. Ed. 2018, 57, 14773–14777.
- 23Y. Gu, Z. Qiu, K. Müllen, J. Am. Chem. Soc. 2022, 144, 11499–11524.
- 24R. E. Blackwell, F. Zhao, E. Brooks, J. Zhu, I. Piskun, S. Wang, A. Delgado, Y.-L. Lee, S. G. Louie, F. R. Fischer, Nature 2021, 600, 647–652.
- 25F. Klappenberger, Y.-Q. Zhang, J. Björk, S. Klyatskaya, M. Ruben, J. V. Barth, Acc. Chem. Res. 2015, 48, 2140–2150.
- 26X. Li, H. Zhang, L. Chi, Adv. Mater. 2019, 31, 1804087.
- 27J. Castro-Esteban, F. Albrecht, S. Fatayer, D. Pérez, L. Gross, D. Peña, Angew. Chem. 2021, 133, 26550–26554.
10.1002/ange.202110311 Google Scholar
- 28C. Huang, Y. Li, N. Wang, Y. Xue, Z. Zuo, H. Liu, Y. Li, Chem. Rev. 2018, 118, 7744–7803.
- 29H. Zhang, C. Song, Y. Lyu, P. Cheng, L. Chen, C. Zhang, S. Meng, K. Wu, Y.-Q. Zhang, Surf. Sci. 2023, 727, 122180.
- 30I. V. Alabugin, E. Gonzalez-Rodriguez, Acc. Chem. Res. 2018, 51, 1206–1219.
- 31S. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo, W. R. Dichtel, Acc. Chem. Res. 2017, 50, 2776–2788.
- 32A. S. Pankova, A. N. Shestakov, M. A. Kuznetsov, Russ. Chem. Rev. 2019, 88, 594–643.
- 33A. D. Senese, W. A. Chalifoux, Molecules 2019, 24, 118.
- 34L. F. Tietze, Chem. Rev. 1996, 96, 115–136.
- 35H. A. Dondas, M. A. D. G. Retamosa, J. M. Sansano, Organometal. 2019, 38, 1828–1867.
- 36K. Fuchibe, Y. Mayumi, N. Zhao, S. Watanabe, M. Yokota, J. Ichikawa, Angew. Chem. Int. Ed. 2013, 52, 7825–7828.
- 37X. Xiao, T. R. Hoye, Nat. Chem. 2018, 10, 838–844.
- 38A. Criado, D. Peña, A. Cobas, E. Guitián, Chem. - Eur. J. 2010, 16, 9736–9740.
- 39W.-J. Kong, Z. Shen, L. H. Finger, L. Ackermann, Angew. Chem. Int. Ed. 2020, 59, 5551–5556.
- 40A.-K. Steiner, K. Y. Amsharov, Angew. Chem. Int. Ed. 2017, 56, 14732–14736.
- 41M. Kolmer, R. Zuzak, A. K. Steiner, L. Zajac, M. Engelund, S. Godlewski, M. Szymonski, K. Amsharov, Science 2019, 363, 57–60.
- 42D. M. Coles, Q. Chen, L. C. Flatten, J. M. Smith, K. Müllen, A. Narita, D. G. Lidzey, Nano Lett. 2017, 17, 5521–5525.
- 43D. Lungerich, O. Papaianina, M. Feofanov, J. Liu, M. Devarajulu, S. I. Troyanov, S. Maier, K. Amsharov, Nat. Commun. 2018, 9, 4756.
- 44Q. Chen, S. Thoms, S. Stöttinger, D. Schollmeyer, K. Müllen, A. Narita, T. Basché, J. Am. Chem. Soc. 2019, 141, 16439–16449.
- 45A. Berdonces-Layunta, J. Lawrence, S. Edalatmanesh, J. Castro-Esteban, T. Wang, M. S. Mohammed, L. Colazzo, D. Pena, P. Jelínek, D. G. de Oteyza, ACS Nano 2021, 15, 5610–5617.
- 46A. Riss, A. P. Paz, S. Wickenburg, H.-Z. Tsai, D. G. de Oteyza, A. J. Bradley, M. M. Ugeda, P. Gorman, H. S. Jung, M. F. Crommie, A. Rubio, F. R. Fischer, Nat. Chem. 2016, 8, 678–683.
- 47B. Cirera, A. Sánchez-Grande, B. de la Torre, J. Santos, S. Edalatmanesh, E. Rodríguez-Sánchez, K. Lauwaet, B. Mallada, R. Zbořil, R. Miranda, O. Gröning, P. Jelínek, N. Martín, D. Ecija, Nat. Nanotechnol. 2020, 15, 437–443.
- 48J. I. Urgel, M. Di Giovannantonio, K. Eimre, T. G. Lohr, J. Liu, S. Mishra, Q. Sun, A. Kinikar, R. Widmer, S. Stolz, M. Bommert, R. Berger, P. Ruffieux, C. A. Pignedoli, K. Müllen, X. Feng, R. Fasel, Angew. Chem. Int. Ed. 2020, 59, 13281–13287.
- 49C. Martín-Fuentes, J. I. Urgel, S. Edalatmanesh, E. Rodríguez-Sánchez, J. Santos, P. Mutombo, K. Biswas, K. Lauwaet, J. M. Gallego, R. Miranda, P. Jelínek, N. Martín, D. Écija, Chem. Commun. 2021, 57, 7545–7548.
- 50D. M. Coles, Q. Chen, L. C. Flatten, J. M. Smith, K. Müllen, A. Narita, D. G. Lidzey, Nano Lett. 2017, 17, 5521–5525.
- 51I. Horcas, R. Fernández, J. Gomez-Rodriguez, J. Colchero, J. Gómez-Herrero, A. M. Baro, Rev. Sci. Instrum. 2007, 78, 013705.
- 52G. Kresse, J. Furthmüller, Comput. Mater. Sci. 1996, 6, 15–50.
- 53J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.