A Self-Assembled Capsule for Propylene/Propane Separation
Chuang-Wei Zhou
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xue-Zhi Wang
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Mo Xie
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorRi-Qin Xia
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorDr. Dong Luo
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorZhao-Xia Lian
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Guo-Hong Ning
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Weigang Lu
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Ping Zhou
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Dan Li
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorChuang-Wei Zhou
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xue-Zhi Wang
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Mo Xie
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorRi-Qin Xia
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorDr. Dong Luo
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorZhao-Xia Lian
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Guo-Hong Ning
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Weigang Lu
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Ping Zhou
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorProf. Dr. Dan Li
College of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632 P. R. China
Search for more papers by this authorGraphical Abstract
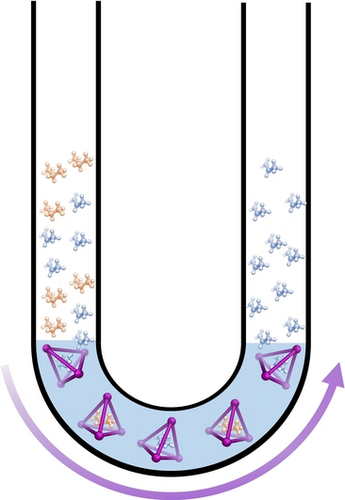
A water-soluble tetrahedral Fe4L6 metal-organic cage has been successfully used to separate propane and propylene under ambient conditions. Mixed gases of C3H6 and C3H8 were captured by Fe4L6 at the gas-liquid interface in a U-shaped glass tube. Governed by the guest binding affinity, C3H6 is released first after transport of the gases to the receiving arm of the tube.
Abstract
The development of energy-saving technology for the efficient separation of olefin and paraffin is highly important for the chemical industry. Herein, we report a self-assembled Fe4L6 capsule containing a hydrophobic cavity, which can be used to encapsulate and separate propylene/propane. The successful encapsulation of propylene and propane by the Fe4L6 cage in a water solution was documented by NMR spectroscopy. The binding constants K for the Fe4L6 cage toward propylene and propane were determined to be (5.0±0.1)×103 M−1 and (2.1±0.7)×104 M−1 in D2O at 25 °C, respectively. Experiments and theoretical studies revealed that the cage exhibited multiple weak interactions with propylene and propane. The polymer-grade propylene (>99.5 %) can be obtained from a mixture of propylene and propane by using the Fe4L6 cage as a separation material in a U-shaped glass tube. This work provides a new strategy for the separation of olefin/paraffin.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202315020-sup-0001-cage_-_gas.cif14.6 MB | Supporting Information |
| anie202315020-sup-0001-misc_information.pdf6.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. S. Sholl, R. P. Lively, Nature 2016, 532, 435–437.
- 2A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout, M. Eddaoudi, Science 2016, 353, 137–140.
- 3
- 3aH. S. Papastathopoulou, W. L. J. I. Luyben, E. C. Research, Ind. Eng. Chem. Res. 1991, 30, 705–713;
- 3bE. Worrell, D. Phylipsen, D. Einstein, N. Martin, Lawrence Berkeley National Lab.(LBNL), Berkeley, CA (United States), 2000;
- 3cK. Li, M. Beaver, Rive Technology, Inc, 2012.
- 4
- 4aR. P. Lively, D. S. Sholl, Nat. Mater. 2017, 16, 276–279;
- 4bL. Yang, S. Qian, X. Wang, X. Cui, B. Chen, H. Xing, Chem. Soc. Rev. 2020, 49, 5359–5406.
- 5J. Wang, C. Ma, J. Liu, Y. Liu, X. Xu, M. Xie, H. Wang, L. Wang, P. Guo, Z. Liu, J. Am. Chem. Soc. 2023, 145, 6853–6860.
- 6E. Perez-Botella, S. Valencia, F. Rey, Chem. Rev. 2022, 122, 17647–17695.
- 7S. Xu, R.-S. Liu, M.-Y. Zhang, A.-H. Lu, Chin. J. Chem. Eng. 2022, 42, 130–150.
- 8H. Wang, Y. Liu, J. Li, Adv. Mater. 2020, 32, 2002603.
- 9H. Jiang, Y. Chen, S. Song, Z. Guo, Z. Zhang, C. Zheng, G. He, H. Wang, H. Wu, T. Huang, Y. Ren, X. Liu, J. Zhang, Y. Yin, Z. Jiang, M. D. Guiver, Chem. Eng. J. 2022, 439, 135657.
- 10J. Gao, Y. Cai, X. Qian, P. Liu, H. Wu, W. Zhou, D. X. Liu, L. Li, R. B. Lin, B. Chen, Angew. Chem. Int. Ed. 2021, 60, 20400–20406.
- 11H. Zeng, M. Xie, T. Wang, R. J. Wei, X. J. Xie, Y. Zhao, W. Lu, D. Li, Nature 2021, 595, 542–548.
- 12
- 12aX. Shen, R. Abro, I. A. Alhumaydhi, A. A. Abdeltawab, A. M. Al-Enizi, X. Chen, G. Yu, Sep. Purif. Technol. 2017, 175, 177–184;
- 12bP. Chilukuri, K. Rademakers, K. Nymeijer, L. van der Ham, H. van den Berg, Ind. Eng. Chem. Res. 2007, 46, 8701–8709;
- 12cW. W. Ho, G. Doyle, D. W. Savage, R. L. Pruett, Ind. Eng. Chem. Res. 1988, 27, 334–337.
- 13
- 13aA. V. Leontiev, A. W. Saleh, D. M. Rudkevich, Org. Lett. 2007, 9, 1753–1755;
- 13bK. E. Chaffee, H. A. Fogarty, T. Brotin, B. M. Goodson, J. P. Dutasta, J. Phys. Chem. A 2009, 113, 13675–13684;
- 13cS. Tartaggia, A. Scarso, P. Padovan, O. De Lucchi, F. Fabris, Org. Lett. 2009, 11, 3926–3929;
- 13dM. Florea, W. M. Nau, Angew. Chem. Int. Ed. 2011, 50, 9338–9342;
- 13eY. Ruan, P. W. Peterson, C. M. Hadad, J. D. Badjic, Chem. Commun. 2014, 50, 9086–9089;
- 13fS. J. Barrow, K. I. Assaf, A. Palma, W. M. Nau, O. A. Scherman, Chem. Sci. 2019, 10, 10240–10246.
- 14
- 14aN. Branda, R. Wyler, J. Rebek Jr., Science 1994, 263, 1267–1268;
- 14bJ. Nakazawa, M. Mizuki, Y. Shimazaki, F. Tani, Y. Naruta, Org. Lett. 2006, 8, 4275–4278.
- 15
- 15aL.-J. Wang, X. Li, S. Bai, Y.-Y. Wang, Y.-F. Han, J. Am. Chem. Soc. 2020, 142, 2524–2531;
- 15bJ. L. Zhu, D. Zhang, T. Ronson, W. Wang, L. Xu, H. Yang, J. R. Nitschke, Angew. Chem. Int. Ed. 2021, 60, 11789–11792;
- 15cM.-Y. Sun, M. Xie, C.-W. Zhou, X.-Z. Wang, Z.-X. Lian, Z.-Y. Chen, Y.-L. Huang, X.-P. Zhou, D. Li, Sci. China Chem. 2023, 66, 2004–2010;
- 15dZ.-E. Zhang, Y.-F. Zhang, Y.-Z. Zhang, H.-L. Li, L.-Y. Sun, L.-J. Wang, Y.-F. Han, J. Am. Chem. Soc. 2023, 145, 7446–7453.
- 16
- 16aO. Barreda, G. Bannwart, G. P. A. Yap, E. D. Bloch, ACS Appl. Mater. Interfaces 2018, 10, 11420–11424;
- 16bH. Han, L. Kan, P. Li, G. Zhang, K. Li, W. Liao, Y. Liu, W. Chen, C. T. Hu, Sci. China Chem. 2021, 64, 426–431.
- 17P. Mal, D. Schultz, K. Beyeh, K. Rissanen, J. R. Nitschke, Angew. Chem. Int. Ed. 2008, 47, 8297–8301.
- 18Deposition numbers 2269782 (for 1), 2269783 (for C3H6@1) and 2269784 (for C3H8@1) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 19P. Mal, B. Breiner, K. Rissanen, J. R. Nitschke, Science 2009, 324, 1697–1699.
- 20M. M. Smulders, S. Zarra, J. R. Nitschke, J. Am. Chem. Soc. 2013, 135, 7039–7046.
- 21I. A. Riddell, M. M. Smulders, J. K. Clegg, J. R. Nitschke, Chem. Commun. 2011, 47, 457–459.
- 22J. Roukala, J. Zhu, C. Giri, K. Rissanen, P. Lantto, V. V. Telkki, J. Am. Chem. Soc. 2015, 137, 2464–2467.
- 23S. Mecozzi, J. J. Rebek, Chem. Eur. J. 1998, 4, 1016–1022.
10.1002/(SICI)1521-3765(19980615)4:6<1016::AID-CHEM1016>3.0.CO;2-B CAS Web of Science® Google Scholar
- 24C. L. Gibb, B. C. Gibb, J. Am. Chem. Soc. 2006, 128, 16498–16499.