Urea-Functionalized Fe4L6 Cages for Supramolecular Gold Catalyst Encapsulation to Control Substrate Activation Modes
Graphical Abstract
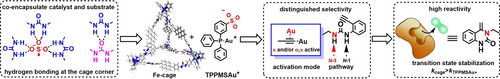
A sulfonate-functionalized gold catalyst and urea substrate have been found to be encapsulated in a urea-functionalized Fe4L6 cage through hydrogen bonding. The caged catalyst presents excellent performances compared to the free catalyst, with high selectivity obtained from the site-isolation effect imposed by the cage only allowing catalysis by π activation of the substrate. The high reactivity arises from stabilization of the transition state.
Abstract
The excellent catalytic performances of enzymes in terms of activity and selectivity are an inspiration for synthetic chemists and this has resulted in the development of synthetic containers for supramolecular catalysis. In such containers the local environment and pre-organization of catalysts and substrates leads to control of the activity and selectivity of the catalyst. Herein we report a supramolecular strategy to encapsulate single catalysts in a urea-functionalized Fe4L6 cage, which can co-encapsulate a functionalized urea substrate through hydrogen bonding. Distinguished selectivity is obtained, imposed by the cage as site isolation only allows catalysis through π activation of the substrate and as a result the selectivity is independent of catalyst concentration. The encapsulated catalyst is more active than the free analogue, an effect that can be ascribed to transitionstate stabilization rather than substrate pre-organization, as revealed by the MM kinetic data. The simple strategy reported here is expected to be of general use in many reactions, for which the catalyst can be functionalized with a sulfonate group required for encapsulation.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.