NH4+ Charge Carrier Coordinated H-Bonded Organic Small Molecule for Fast and Superstable Rechargeable Zinc Batteries
Dr. Ziyang Song
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorDr. Ling Miao
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorDr. Yaokang Lv
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorProf. Lihua Gan
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Mingxian Liu
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorDr. Ziyang Song
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorDr. Ling Miao
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorDr. Yaokang Lv
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorProf. Lihua Gan
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Mingxian Liu
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092 P. R. China
Search for more papers by this authorGraphical Abstract
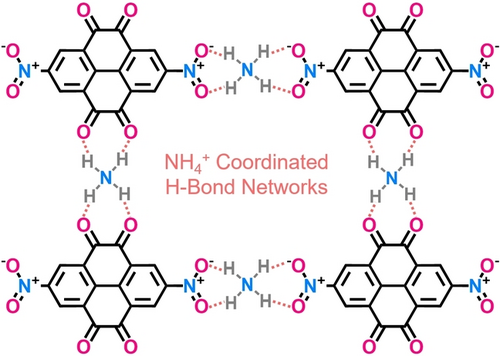
Ultrastable lock-and-key H-bonding networks between octuple-active 2, 7-dinitropyrene-4, 5, 9, 10-tetraone and tetrahedral NH4+ charge carrier are designed, which conquers its stability barrier in aqueous electrolyte and achieves fast diffusion kinetics of non-metallic charge carrier. A unique two-step four-electron NH4+ coordination mechanism brings the Zn-organic battery high capacity, superior rate capability and superstable cyclability.
Abstract
Organic small molecules as high-capacity cathodes for Zn-organic batteries have inspired numerous interests, but are trapped by their easy-dissolution in electrolytes. Here we knit ultrastable lock-and-key hydrogen-bonding networks between 2, 7-dinitropyrene-4, 5, 9, 10-tetraone (DNPT) and NH4+ charge carrier. DNPT with octuple-active carbonyl/nitro centers (H-bond acceptor) are redox-exclusively accessible for flexible tetrahedral NH4+ ions (H-bond donator) but exclude larger and rigid Zn2+, due to a lower activation energy (0.14 vs. 0.31 eV). NH4+ coordinated H-bonding chemistry conquers the stability barrier of DNPT in electrolyte, and gives fast diffusion kinetics of non-metallic charge carrier. A stable two-step 4e− NH4+ coordination with DNPT cathode harvests a high capacity (320 mAh g−1), a high-rate capability (50 A g−1) and an ultralong life (60,000 cycles). This finding points to a new paradigm for H-bond stabilized organic small molecules to design advanced zinc batteries.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202309446-sup-0001-misc_information.pdf4.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aZ. Tie, Y. Zhang, J. Zhu, S. Bi, Z. Niu, J. Am. Chem. Soc. 2022, 144, 10301;
- 1bQ. Zhao, W. Huang, Z. Luo, L. Liu, Y. Lu, Y. Li, L. Li, J. Hu, H. Ma, J. Chen, Sci. Adv. 2018, 4, eaao1761;
- 1cX. Qiu, J. Xu, K. Zhou, H. Xin, M. Liao, Y. Cao, G. Zhou, P. Wei, Y. Wang, Angew. Chem. Int. Ed. 2023, 62, e202304036;
- 1dZ. Xu, M. Li, W. Sun, T. Tang, J. Lu, X. Wang, Adv. Mater. 2022, 34, 2200077;
- 1eZ. Sang, J. Liu, X. Zhang, L. Yin, F. Hou, J. Liang, ACS Nano 2023, 17, 3077;
- 1fP. Canepa, G. Sai Gautam, D. C. Hannah, R. Malik, M. Liu, K. G. Gallagher, K. A. Persson, G. Ceder, Chem. Rev. 2017, 117, 4287.
- 2
- 2aF. Xie, H. Li, X. Wang, X. Zhi, D. Chao, K. Davey, S. Z. Qiao, Adv. Energy Mater. 2021, 11, 2003419;
- 2bF. Ye, Q. Liu, H. Dong, K. Guan, Z. Chen, N. Ju, L. Hu, Angew. Chem. Int. Ed. 2022, 61, e202214244;
- 2cZ. Lin, H.-Y. Shi, L. Lin, X. Yang, W. Wu, X. Sun, Nat. Commun. 2021, 12, 4424;
- 2dC.-X. Zhao, J.-N. Liu, N. Yao, J. Wang, D. Ren, X. Chen, B.-Q. Li, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 15281.
- 3
- 3aN. Patil, C. Cruz, D. Ciurduc, A. Mavrandonakis, J. Palma, R. Marcilla, Adv. Energy Mater. 2021, 11, 2100939;
- 3bQ.-Q. Sun, T. Sun, J.-Y. Du, K. Li, H.-M. Xie, G. Huang, X.-B. Zhang, Adv. Mater. 2023, 35, 2301088;
- 3cZ. Li, J. Tan, Y. Wang, C. Gao, Y. Wang, M. Ye, J. Shen, Energy Environ. Sci. 2023, 16, 2398;
- 3dW. Sun, C. Zhou, Y. Fan, Y. He, H. Zhang, Z. Quan, H. Kong, F. Fu, J. Qin, Y. Shen, H. Chen, Angew. Chem. Int. Ed. 2023, 62, e202300158.
- 4
- 4aH. Cui, T. Wang, Z. Huang, G. Liang, Z. Chen, A. Chen, D. Wang, Q. Yang, H. Hong, J. Fan, C. Zhi, Angew. Chem. Int. Ed. 2022, 61, e202203453;
- 4bH. Zhang, Y. Fang, F. Yang, X. Liu, X. Lu, Energy Environ. Sci. 2020, 13, 2515.
- 5
- 5aY. Gao, G. Li, F. Wang, J. Chu, P. Yu, B. Wang, H. Zhan, Z. Song, Energy Storage Mater. 2021, 40, 31;
- 5bH. Zhang, L. Zhong, J. Xie, F. Yang, X. Liu, X. Lu, Adv. Mater. 2021, 33, 2101857.
- 6
- 6aK. W. Nam, H. Kim, Y. Beldjoudi, T. W. Kwon, D. J. Kim, J. F. Stoddart, J. Am. Chem. Soc. 2020, 142, 2541;
- 6bS. Li, J. Shang, M. Li, M. Xu, F. Zeng, H. Yin, Y. Tang, C. Han, H. M. Cheng, Adv. Mater. 2022, 34, 202207115.
- 7
- 7aS. Zheng, D. Shi, D. Yan, Q. Wang, T. Sun, T. Ma, L. Li, D. He, Z. Tao, J. Chen, Angew. Chem. Int. Ed. 2022, 61, e202117511;
- 7bJ. Meng, A. Robles, S. Jalife, W. Ren, Y. Zhang, L. Zhao, Y. Liang, J. I. Wu, O. S. Miljanic, Y. Yao, Angew. Chem. Int. Ed. 2023, 62, e202300892.
- 8
- 8aY. Zhao, Y. Huang, F. Wu, R. Chen, L. Li, Adv. Mater. 2021, 33, 202106469;
- 8bZ. Song, L. Miao, H. Duan, L. Ruhlmann, Y. Lv, D. Zhu, L. Li, L. Gan, M. Liu, Angew. Chem. Int. Ed. 2022, 61, e202208821.
- 9
- 9aY. Lu, Q. Zhang, L. Li, Z. Niu, J. Chen, Chem 2018, 4, 2786;
- 9bL. Miao, Z. Song, W. Du, X. Zheng, Y. Lv, L. Gan, M. Liu, Mater. Chem. Front. 2023, 7, 2731.
- 10
- 10aS. Wang, Z. Yuan, X. Zhang, S. Bi, Z. Zhou, J. Tian, Q. Zhang, Z. Niu, Angew. Chem. Int. Ed. 2021, 60, 7056;
- 10bL. Xie, K. Xu, W. Sun, Y. Fan, J. Zhang, Y. Zhang, H. Zhang, J. Chen, Y. Shen, F. Fu, H. Kong, G. Wu, J. Wu, L. Chen, H. Chen, Angew. Chem. Int. Ed. 2023, 62, e202300372;
- 10cY. Lin, H. Cui, C. Liu, R. Li, S. Wang, G. Qu, Z. Wei, Y. Yang, Y. Wang, Z. Tang, H. Li, H. Zhang, C. Zhi, H. Lv, Angew. Chem. Int. Ed. 2023, 62, e202218745.
- 11
- 11aM. Yu, N. Chandrasekhar, R. K. M. Raghupathy, K. H. Ly, H. Zhang, E. Dmitrieva, C. Liang, X. Lu, T. D. Kuhne, H. Mirhosseini, I. M. Weidinger, X. Feng, J. Am. Chem. Soc. 2020, 142, 19570;
- 11bH. Peng, S. Huang, V. Montes-García, D. Pakulski, H. Guo, F. Richard, X. Zhuang, P. Samorì, A. Ciesielski, Angew. Chem. Int. Ed. 2023, 62, e202216136.
- 12
- 12aZ. Tie, L. Liu, S. Deng, D. Zhao, Z. Niu, Angew. Chem. Int. Ed. 2020, 59, 4920;
- 12bZ. Song, L. Miao, L. Ruhlmann, Y. Lv, L. Li, L. Gan, M. Liu, Angew. Chem. Int. Ed. 2023, 62, e202219136;
- 12cY. Chen, H. Dai, K. Fan, G. Zhang, M. Tang, Y. Gao, C. Zhang, L. Guan, M. Mao, H. Liu, T. Zhai, C. Wang, Angew. Chem. Int. Ed. 2023, 62, e202302539.
- 13
- 13aZ. Tian, V. S. Kale, Y. Wang, S. Kandambeth, J. Czaban-Jozwiak, O. Shekhah, M. Eddaoudi, H. N. Alshareef, J. Am. Chem. Soc. 2021, 143, 19178;
- 13bS. Zhang, K. Zhu, Y. Gao, D. Cao, ACS Energy Lett. 2023, 8, 889;
- 13cM. Huang, Q. He, J. Wang, X. Liu, F. Xiong, Y. Liu, R. Guo, Y. Zhao, J. Yang, L. Mai, Angew. Chem. Int. Ed. 2023, 62, e202218922.
- 14
- 14aR. Zhang, S. Wang, S. Chou, H. Jin, Adv. Funct. Mater. 2022, 32, 2112179;
- 14bF. Ye, R. Pang, C. Lu, Q. Liu, Y. Wu, R. Ma, L. Hu, Angew. Chem. Int. Ed. 2023, 62, e202303480.
- 15Y. Chen, J. Li, Q. Zhu, K. Fan, Y. Cao, G. Zhang, C. Zhang, Y. Gao, J. Zou, T. Zhai, C. Wang, Angew. Chem. Int. Ed. 2022, 61, e202116289.
- 16
- 16aL. Lin, Z. Lin, J. Zhu, K. Wang, W. Wu, T. Qiu, X. Sun, Energy Environ. Sci. 2023, 16, 89;
- 16bT. Sun, W. Zhang, Z. Zha, M. Cheng, D. Li, Z. Tao, Energy Storage Mater. 2023, 59, 102778.
- 17
- 17aY. Zhong, Y. Li, J. Meng, X. Lin, Z. Huang, Y. Shen, Y. Huang, Energy Storage Mater. 2021, 43, 492;
- 17bJ. Yang, H. Hua, H. Yang, P. Lai, M. Zhang, Z. Lv, Z. Wen, C. C. Li, J. Zhao, Y. Yang, Adv. Energy Mater. 2023, 13, 2204005.
- 18
- 18aZ. Ye, S. Xie, Z. Cao, L. Wang, D. Xu, H. Zhang, J. Matz, P. Dong, H. Fang, J. Shen, M. Ye, Energy Storage Mater. 2021, 37, 378;
- 18bC. Zhang, W. Ma, C. Han, L.-W. Luo, A. Daniyar, S. Xiang, X. Wu, X. Ji, J.-X. Jiang, Energy Environ. Sci. 2021, 14, 462.
- 19L. Yan, Q. Zhu, Y. Qi, J. Xu, Y. Peng, J. Shu, J. Ma, Y. Wang, Angew. Chem. Int. Ed. 2022, 61, e202211107.
- 20B. Liu, X. Hao, T. Zhai, S. Sun, H. Zhang, P. A. Koudakan, C. Wei, G. Wang, H. Xia, Energy Storage Mater. 2022, 48, 403.
- 21W. Zhang, J. Yin, W. Jian, Y. Wu, L. Chen, M. Sun, U. Schwingenschlögl, X. Qiu, H. N. Alshareef, Nano Energy 2022, 103, 107827.
- 22
- 22aL. Yan, Y. Zhang, Z. Ni, Y. Zhang, J. Xu, T. Kong, J. Huang, W. Li, J. Ma, Y. Wang, J. Am. Chem. Soc. 2021, 143, 15369;
- 22bZ. Song, L. Miao, L. Ruhlmann, Y. Lv, D. Zhu, L. Li, L. Gan, M. Liu, Adv. Funct. Mater. 2022, 32, 2208049;
- 22cY. Zhang, Z. Song, L. Miao, Y. Lv, L. Li, L. Gan, M. Liu, Chem. Eng. J. 2023, 467, 143497.
- 23
- 23aS. Yang, H. Lv, Y. Wang, X. Guo, L. Zhao, H. Li, C. Zhi, Angew. Chem. Int. Ed. 2022, 61, e202209794;
- 23bJ. Xie, F. Yu, J. Zhao, W. Guo, H.-L. Zhang, G. Cui, Q. Zhang, Energy Storage Mater. 2020, 33, 283;
- 23cY. Zhao, R. Zhou, Z. Song, X. Zhang, T. Zhang, A. Zhou, F. Wu, R. Chen, L. Li, Angew. Chem. Int. Ed. 2022, 61, e202212231.
- 24J. Wei, P. Zhang, T. Shen, Y. Liu, T. Dai, Z. Tie, Z. Jin, ACS Energy Lett. 2023, 8, 762.
- 25J. Ming, Z. Cao, Q. Li, W. Wahyudi, W. Wang, L. Cavallo, K.-J. Park, Y.-K. Sun, H. N. Alshareef, ACS Energy Lett. 2019, 4, 1584.
- 26A. Duan, Z. Wang, X. Huang, Y. Li, Angew. Chem. Int. Ed. 2023, 62, e202302754.
- 27N. Wang, R. Zhou, H. Li, Z. Zheng, W. Song, T. Xin, M. Hu, J. Liu, ACS Energy Lett. 2021, 6, 1141.
- 28
- 28aZ. Song, L. Miao, L. Ruhlmann, Y. Lv, D. Zhu, L. Li, L. Gan, M. Liu, Adv. Mater. 2021, 33, 2104148;
- 28bX. Deng, J. K. Sarpong, G. Zhang, J. Hao, X. Zhao, L. Li, H. Li, C. Han, B. Li, InfoMat 2022, 5, e12382.
- 29N. Liu, X. Wu, Y. Zhang, Y. Yin, C. Sun, Y. Mao, L. Fan, N. Zhang, Adv. Sci. 2020, 7, 2000146.
- 30X. Liu, Z. Ye, Adv. Energy Mater. 2021, 11, 2003281.
- 31
- 31aT. Sun, Z. J. Li, Y. F. Zhi, Y. J. Huang, H. J. Fan, Q. Zhang, Adv. Funct. Mater. 2021, 31, 2010049;
- 31bC. Luo, X. Ji, S. Hou, N. Eidson, X. Fan, Y. Liang, T. Deng, J. Jiang, C. Wang, Adv. Mater. 2018, 30, 1706498.
- 32X. Wang, J. Xiao, W. Tang, Adv. Funct. Mater. 2021, 31, 2108225.
- 33X. Wang, Y. Liu, Z. Wei, J. Hong, H. Liang, M. Song, Y. Zhou, X. Huang, Adv. Mater. 2022, 34, 2206812.
- 34N. Zhang, A. Jalil, D. Wu, S. Chen, Y. Liu, C. Gao, W. Ye, Z. Qi, H. Ju, C. Wang, X. Wu, L. Song, J. Zhu, Y. Xiong, J. Am. Chem. Soc. 2018, 140, 9434.
- 35Z. Tie, S. Deng, H. Cao, M. Yao, Z. Niu, J. Chen, Angew. Chem. Int. Ed. 2022, 61, e202115180.
- 36L. Xiang, S. Yuan, F. Wang, Z. Xu, X. Li, F. Tian, L. Wu, W. Yu, Y. Mai, J. Am. Chem. Soc. 2022, 144, 15497.