Metal-DNA Nanocomplexes Enhance Chemo-dynamic Therapy by Inhibiting Autophagy-Mediated Resistance
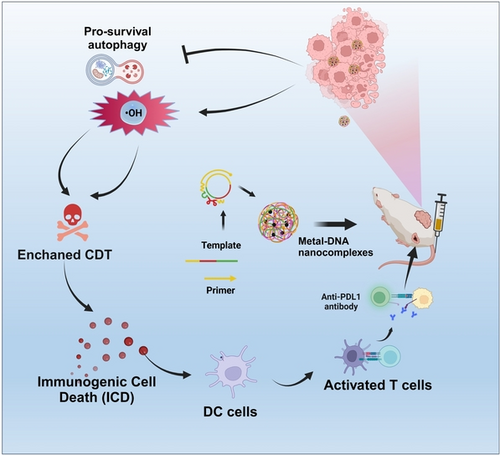
Graphical Abstract
By integrating multivalent nucleic acid aptamers and DNAzyme sequences into long single-stranded DNA and further forming metal-DNA nanocomplexes using rolling circle amplification (RCA) technology, researchers have successfully established a DNA drug delivery platform that combined chemo-dynamic therapy (CDT) and autophagy inhibition therapies. The platform overcame the shortcomings of short-stranded DNA, such as poor stability under physiological conditions, short blood circulation time, and easy clearance by the organism.
Abstract
Chemo-dynamic therapy (CDT) based on the Fenton or Fenton-like reaction has emerged as a promising approach for cancer treatment. However, autophagy-mediated self-protection mechanisms of cancer cells pose a significant challenge to the efficacy of CDT. Herein, we developed metal-DNA nanocomplexes (DACs-Mn) to enhance CDT via DNAzyme inhibition of autophagy. Specifically, Mn-based catalyst in DACs-Mn was used to generate highly hydroxyl radicals (⋅OH) that kill cancer cells, while the ATG5 DNAzyme incorporated into DACs-Mn inhibited the expression of autophagy-associated proteins, thereby improving the efficacy of CDT. By disrupting the self-protective pathway of cells under severe oxidative stress, this novel approach of DACs-Mn was found to synergistically enhance CDT in both in vitro and in vivo models, effectively amplifying tumor-specific oxidative damage. Notably, the Metal-DNA nanocomplexes can also induce immunogenic cell death (ICD), thereby inhibiting tumor metastasis. Specifically, in a bilateral tumor model in mice, the combined approach of CDT and autophagy inhibition followed by immune checkpoint blockade therapy shown significant potential as a novel and effective treatment modality for primary and metastatic tumors.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.