Organogold(III) Complexes Display Conditional Photoactivities: Evolving From Photodynamic into Photoactivated Chemotherapy in Response to O2 Consumption for Robust Cancer Therapy
Yunli Luo
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorDr. Bei Cao
Warshel Institute for Computational Biology, and General Education Division, The Chinese University of Hong Kong, Shenzhen, 518172 P. R. China
Search for more papers by this authorMingjie Zhong
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorMoyi Liu
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorDr. Xiaolin Xiong
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Taotao Zou
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorYunli Luo
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorDr. Bei Cao
Warshel Institute for Computational Biology, and General Education Division, The Chinese University of Hong Kong, Shenzhen, 518172 P. R. China
Search for more papers by this authorMingjie Zhong
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorMoyi Liu
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorDr. Xiaolin Xiong
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Taotao Zou
Guangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, 510006 P. R. China
Search for more papers by this authorGraphical Abstract
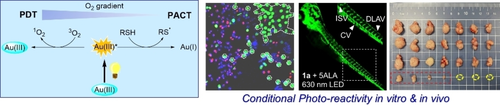
Oxygen-dependent photo-reactivity for robust tumor therapy: A PDT-to-PACT evolving photo-reactivity with response to oxygen-consumption was found in cyclometalated gold(III)-alkyne complexes, leading to potent cytotoxicity towards cancer cells, strong anti-angiogenesis in zebrafish, and efficient suppression of mouse tumor xenografts.
Abstract
Photodynamic therapy (PDT) is a spatiotemporally controllable, powerful approach in combating cancers but suffers from low activity under hypoxia, whereas photoactivated chemotherapy (PACT) operates in an O2-independent manner but compromises the ability to harness O2 for potent photosensitization. Herein we report that cyclometalated gold(III)-alkyne complexes display a PDT-to-PACT evolving photoactivity for efficient cancer treatment. On the one hand, the gold(III) complexes can act as dual photosensitizers and substrates, leading to conditional PDT activity in oxygenated condition that progresses to highly efficient PACT (ϕ up to 0.63) when O2 is depleted in solution and under cellular environment. On the other hand, the conditional PDT-to-PACT reactivity can be triggered by external photosensitizers in a similar manner in vitro and in vivo, giving additional tumor-selectivity and/or deep tissue penetration by red-light irradiation that leads to robust anticancer efficacy.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202212689-sup-0001-misc_information.pdf3.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Shi, S. Monro, R. Hennigar, J. Colpitts, J. Fong, K. Kasimova, H. Yin, R. DeCoste, C. Spencer, L. Chamberlain, A. Mandel, L. Lilge, S. A. McFarland, Coord. Chem. Rev. 2015, 282–283, 127–138;
- 1bS. Monro, K. L. Colon, H. Yin, J. Roque III, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron, S. A. McFarland, Chem. Rev. 2019, 119, 797–828;
- 1cH. Shi, P. J. Sadler, Br. J. Cancer 2020, 123, 871–873;
- 1dP. C. Lo, M. S. Rodriguez-Morgade, R. K. Pandey, D. K. P. Ng, T. Torres, F. Dumoulin, Chem. Soc. Rev. 2020, 49, 1041–1056;
- 1eJ. Karges, Angew. Chem. Int. Ed. 2022, 61, e202112236; Angew. Chem. 2022, 134, e202112236;
- 1fK. A. Ryu, C. M. Kaszuba, N. B. Bissonnette, R. C. Oslund, O. O. Fadeyi, Nat. Chem. Rev. 2021, 5, 322–337;
- 1gT. C. Pham, V. N. Nguyen, Y. Choi, S. Lee, J. Yoon, Chem. Rev. 2021, 121, 13454–13619.
- 2
- 2aZ. Zhou, J. Song, L. Nie, X. Chen, Chem. Soc. Rev. 2016, 45, 6597–6626;
- 2bF. Heinemann, J. Karges, G. Gasser, Acc. Chem. Res. 2017, 50, 2727–2736;
- 2cJ. Xie, Y. Wang, W. Choi, P. Jangili, Y. Ge, Y. Xu, J. Kang, L. Liu, B. Zhang, Z. Xie, J. He, N. Xie, G. Nie, H. Zhang, J. S. Kim, Chem. Soc. Rev. 2021, 50, 9152–9201;
- 2dX. Zhao, J. Liu, J. Fan, H. Chao, X. Peng, Chem. Soc. Rev. 2021, 50, 4185–4219.
- 3
- 3aJ. M. Brown, W. R. Wilson, Nat. Rev. Cancer 2004, 4, 437–447;
- 3bW. Zeng, P. Liu, W. Pan, S. R. Singh, Y. Wei, Cancer Lett. 2015, 356, 263–267.
- 4F. Wei, T. W. Rees, X. Liao, L. Ji, H. Chao, Coord. Chem. Rev. 2021, 432, 213714.
- 5
- 5aH. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao, P. J. Sadler, Nat. Chem. 2019, 11, 1041–1048;
- 5bJ. Du, T. Shi, S. Long, P. Chen, W. Sun, J. Fan, X. Peng, Coord. Chem. Rev. 2021, 427, 213604;
- 5cL. Huang, S. Zhao, J. Wu, L. Yu, N. Singh, K. Yang, M. Lan, P. Wang, J. S. Kim, Coord. Chem. Rev. 2021, 438, 213888;
- 5dL. Gourdon, K. Cariou, G. Gasser, Chem. Soc. Rev. 2022, 51, 1167–1195.
- 6
- 6aN. J. Farrer, L. Salassa, P. J. Sadler, Dalton Trans. 2009, 10690–10701;
- 6bX. Wang, X. Wang, S. Jin, N. Muhammad, Z. Guo, Chem. Rev. 2019, 119, 1138–1192;
- 6cS. Bonnet, Dalton Trans. 2018, 47, 10330–10343;
- 6dC. Imberti, P. Zhang, H. Huang, P. J. Sadler, Angew. Chem. Int. Ed. 2020, 59, 61–73; Angew. Chem. 2020, 132, 61–73;
- 6eR. Weinstain, T. Slanina, D. Kand, P. Klán, Chem. Rev. 2020, 120, 13135–13272;
- 6fM. Martínez-Alonso, G. Gasser, Coord. Chem. Rev. 2021, 434, 213736.
- 7
- 7aF. S. Mackay, J. A. Woods, H. Moseley, J. Ferguson, A. Dawson, S. Parsons, P. J. Sadler, Chem. Eur. J. 2006, 12, 3155–3161;
- 7bN. J. Farrer, J. A. Woods, L. Salassa, Y. Zhao, K. S. Robinson, G. Clarkson, F. S. Mackay, P. J. Sadler, Angew. Chem. Int. Ed. 2010, 49, 8905–8908; Angew. Chem. 2010, 122, 9089–9092;
- 7cF. Barragán, P. Lopez-Senin, L. Salassa, S. Betanzos-Lara, A. Habtemariam, V. Moreno, P. J. Sadler, V. Marchan, J. Am. Chem. Soc. 2011, 133, 14098–14108;
- 7dB. S. Howerton, D. K. Heidary, E. C. Glazer, J. Am. Chem. Soc. 2012, 134, 8324–8327;
- 7eM. A. Sgambellone, A. David, R. N. Garner, K. R. Dunbar, C. Turro, J. Am. Chem. Soc. 2013, 135, 11274–11282;
- 7fT. Joshi, V. Pierroz, C. Mari, L. Gemperle, S. Ferrari, G. Gasser, Angew. Chem. Int. Ed. 2014, 53, 2960–2963; Angew. Chem. 2014, 126, 3004–3007;
- 7gB. A. Albani, B. Peña, N. A. Leed, N. A. B. G. de Paula, C. Pavani, M. S. Baptista, K. R. Dunbar, C. Turro, J. Am. Chem. Soc. 2014, 136, 17095–17101;
- 7hL. N. Lameijer, D. Ernst, S. L. Hopkins, M. S. Meijer, S. H. C. Askes, S. E. Le Dévédec, S. Bonnet, Angew. Chem. Int. Ed. 2017, 56, 11549–11553; Angew. Chem. 2017, 129, 11707–11711;
- 7iK. Arora, M. Herroon, M. H. Al-Afyouni, N. P. Toupin, T. N. Rohrabaugh, L. M. Loftus, I. Podgorski, C. Turro, J. J. Kodanko, J. Am. Chem. Soc. 2018, 140, 14367–14380;
- 7jS. Alonso-de Castro, A. L. Cortajarena, F. López-Gallego, L. Salassa, Angew. Chem. Int. Ed. 2018, 57, 3143–3147; Angew. Chem. 2018, 130, 3197–3201;
- 7kZ. Wang, N. Wang, S.-C. Cheng, K. Xu, Z. Deng, S. Chen, Z. Xu, K. Xie, M.-K. Tse, P. Shi, H. Hirao, C.-C. Ko, G. Zhu, Chem 2019, 5, 3151–3165;
- 7lL. N. Lameijer, C. van de Griend, S. L. Hopkins, A.-G. Volbeda, S. H. C. Askes, M. A. Siegler, S. Bonnet, J. Am. Chem. Soc. 2019, 141, 352–362;
- 7mV. H. S. van Rixel, V. Ramu, A. B. Auyeung, N. Beztsinna, D. Y. Leger, L. N. Lameijer, S. T. Hilt, S. E. Le Dévédec, T. Yildiz, T. Betancourt, M. B. Gildner, T. W. Hudnall, V. Sol, B. Liagre, A. Kornienko, S. Bonnet, J. Am. Chem. Soc. 2019, 141, 18444–18454;
- 7nZ. Deng, N. Wang, Y. Liu, Z. Xu, Z. Wang, T. C. Lau, G. Zhu, J. Am. Chem. Soc. 2020, 142, 7803–7812;
- 7oJ. Karges, T. Yempala, M. Tharaud, D. Gibson, G. Gasser, Angew. Chem. Int. Ed. 2020, 59, 7069–7075; Angew. Chem. 2020, 132, 7135–7141;
- 7pG. Thiabaud, R. McCall, G. He, J. F. Arambula, Z. H. Siddik, J. L. Sessler, Angew. Chem. Int. Ed. 2016, 55, 12626–12631; Angew. Chem. 2016, 128, 12816–12821.
- 8
- 8aJ. D. Knoll, B. A. Albani, C. Turro, Acc. Chem. Res. 2015, 48, 2280–2287;
- 8bH. D. Cole, J. A. Roque III, G. Shi, L. M. Lifshits, E. Ramasamy, P. C. Barrett, R. O. Hodges, C. G. Cameron, S. A. McFarland, J. Am. Chem. Soc. 2022, 144, 9543–9547;
- 8cN. Toupin, S. J. Steinke, S. Nadella, A. Li, T. N. Rohrabaugh, E. R. Samuels, C. Turro, I. F. Sevrioukova, J. J. Kodanko, J. Am. Chem. Soc. 2021, 143, 9191–9205.
- 9D. K. Kölmel, R. P. Loach, T. Knauber, M. E. Flanagan, ChemMedChem 2018, 13, 2159–2165.
- 10
- 10aW. P. To, K. T. Chan, G. S. Tong, C. Ma, W. M. Kwok, X. Guan, K. H. Low, C. M. Che, Angew. Chem. Int. Ed. 2013, 52, 6648–6652; Angew. Chem. 2013, 125, 6780–6784;
- 10bR. Kumar, C. Nevado, Angew. Chem. Int. Ed. 2017, 56, 1994–2015; Angew. Chem. 2017, 129, 2024–2046;
- 10cP. J. Barnard, L. E. Wedlock, M. V. Baker, S. J. Berners-Price, D. A. Joyce, B. W. Skelton, J. H. Steer, Angew. Chem. Int. Ed. 2006, 45, 5966–5970; Angew. Chem. 2006, 118, 6112–6116;
- 10dI. Ott, Coord. Chem. Rev. 2009, 253, 1670–1681;
- 10eA. Meyer, C. P. Bagowski, M. Kokoschka, M. Stefanopoulou, H. Alborzinia, S. Can, D. H. Vlecken, W. S. Sheldrick, S. Wölfl, I. Ott, Angew. Chem. Int. Ed. 2012, 51, 8895–8899; Angew. Chem. 2012, 124, 9025–9030;
- 10fS. J. Berners-Price, P. J. Barnard, in Ligand Design in Medicinal Inorganic Chemistry, Wiley, Hoboken, 2014, pp. 227–256;
10.1002/9781118697191.ch9 Google Scholar
- 10gT. Zou, C. T. Lum, C. N. Lok, J. J. Zhang, C. M. Che, Chem. Soc. Rev. 2015, 44, 8786–8801;
- 10hJ. Fernández-Gallardo, B. T. Elie, T. Sadhukha, S. Prabha, M. Sanaú, S. A. Rotenberg, J. W. Ramos, M. Contel, Chem. Sci. 2015, 6, 5269–5283;
- 10iC. Bazzicalupi, M. Ferraroni, F. Papi, L. Massai, B. Bertrand, L. Messori, P. Gratteri, A. Casini, Angew. Chem. Int. Ed. 2016, 55, 4256–4259; Angew. Chem. 2016, 128, 4328–4331;
- 10jD. Wragg, A. de Almeida, R. Bonsignore, F. E. Kühn, S. Leoni, A. Casini, Angew. Chem. Int. Ed. 2018, 57, 14524–14528; Angew. Chem. 2018, 130, 14732–14736;
- 10kA. Casini, R. W.-Y. Sun, I. Ott, in Metallo-Drugs: Development and Action of Anticancer Agents (Eds.: S. Astrid, S. Helmut, F. Eva, K. O. S. Roland), De Gruyter, Berlin, 2018, pp. 199–218;
- 10lM. Mora, M. C. Gimeno, R. Visbal, Chem. Soc. Rev. 2019, 48, 447–462;
- 10mC. Zhang, P.-Y. Fortin, G. Barnoin, X. Qin, X. Wang, A. Fernandez Alvarez, C. Bijani, M.-L. Maddelein, C. Hemmert, O. Cuvillier, H. Gornitzka, Angew. Chem. Int. Ed. 2020, 59, 12062–12068; Angew. Chem. 2020, 132, 12160–12166;
- 10nM. Fares, X. Wu, D. Ramesh, W. Lewis, P. A. Keller, E. N. W. Howe, R. Pérez-Tomás, P. A. Gale, Angew. Chem. Int. Ed. 2020, 59, 17614–17621; Angew. Chem. 2020, 132, 17767–17774;
- 10oJ.-J. Zhang, M. A. Abu el Maaty, H. Hoffmeister, C. Schmidt, J. K. Muenzner, R. Schobert, S. Wölfl, I. Ott, Angew. Chem. Int. Ed. 2020, 59, 16795–16800; Angew. Chem. 2020, 132, 16940–16945;
- 10pR. P. Herrera, M. C. Gimeno, Chem. Rev. 2021, 121, 8311–8363;
- 10qT. Gamberi, A. Pratesi, L. Messori, L. Massai, Coord. Chem. Rev. 2021, 438, 213905;
- 10rX.-Q. Zhou, I. Carbo-Bague, M. A. Siegler, J. Hilgendorf, U. Basu, I. Ott, R. Liu, L. Zhang, V. Ramu, A. P. Ijzerman, S. Bonnet, JACS Au 2021, 1, 380–395;
- 10sM. V. Babak, K. R. Chong, P. Rapta, M. Zannikou, H. M. Tang, L. Reichert, M. R. Chang, V. Kushnarev, P. Heffeter, S. M. Meier-Menches, Z. C. Lim, J. Y. Yap, A. Casini, I. V. Balyasnikova, W. H. Ang, Angew. Chem. Int. Ed. 2021, 60, 13405–13413; Angew. Chem. 2021, 133, 13517–13525;
- 10tJ. Jiang, B. Cao, Y. Chen, H. Luo, J. Xue, X. Xiong, T. Zou, Angew. Chem. Int. Ed. 2022, 61, e202201103; Angew. Chem. 2022, 134, e202201103.
- 11
- 11aH. Luo, B. Cao, A. S. C. Chan, R. W.-Y. Sun, T. Zou, Angew. Chem. Int. Ed. 2020, 59, 11046–11052; Angew. Chem. 2020, 132, 11139–11145;
- 11bL. Rocchigiani, M. Bochmann, Chem. Rev. 2021, 121, 8364–8451.
- 12S. Wan, W. Lu, Angew. Chem. Int. Ed. 2017, 56, 1784–1788; Angew. Chem. 2017, 129, 1810–1814.
- 13
- 13aF. F. Hung, W. P. To, J. J. Zhang, C. Ma, W. Y. Wong, C. M. Che, Chem. Eur. J. 2014, 20, 8604–8614;
- 13bM.-C. Tang, M.-Y. Chan, V. W.-W. Yam, Chem. Rev. 2021, 121, 7249–7279.
- 14A. Bahreman, J.-A. Cuello-Garibo, S. Bonnet, Dalton Trans. 2014, 43, 4494–4505.
- 15T. Zou, C. T. Lum, S. S.-Y. Chui, C.-M. Che, Angew. Chem. Int. Ed. 2013, 52, 2930–2933; Angew. Chem. 2013, 125, 3002–3005.
- 16
- 16aS. Urig, K. Fritz-Wolf, R. Réau, C. Herold-Mende, K. Tóth, E. Davioud-Charvet, K. Becker, Angew. Chem. Int. Ed. 2006, 45, 1881–1886; Angew. Chem. 2006, 118, 1915–1920;
- 16bS. Ray, R. Mohan, J. K. Singh, M. K. Samantaray, M. M. Shaikh, D. Panda, P. Ghosh, J. Am. Chem. Soc. 2007, 129, 15042–15053;
- 16cJ. L. Hickey, R. A. Ruhayel, P. J. Barnard, M. V. Baker, S. J. Berners-Price, A. Filipovska, J. Am. Chem. Soc. 2008, 130, 12570–12571;
- 16dA. Bindoli, M. P. Rigobello, G. Scutari, C. Gabbiani, A. Casini, L. Messori, Coord. Chem. Rev. 2009, 253, 1692–1707;
- 16eA. Casini, L. Messori, Curr. Top. Med. Chem. 2011, 11, 2647–2660;
- 16fM. Frik, J. Fernández-Gallardo, O. Gonzalo, V. Mangas-Sanjuan, M. González-Alvarez, A. Serrano del Valle, C. Hu, I. González-Alvarez, M. Bermejo, I. Marzo, M. Contel, J. Med. Chem. 2015, 58, 5825–5841;
- 16gA. Giorgio, A. Merlino, Coord. Chem. Rev. 2020, 407, 213175.
- 17W. Chen, J.-J. Chen, R. Lu, C. Qian, W.-W. Li, H.-Q. Yu, Bioelectrochemistry 2014, 98, 103–108.
- 18
- 18aS. Alonso-de Castro, E. Ruggiero, A. Ruiz-de-Angulo, E. Rezabal, J. C. Mareque-Rivas, X. Lopez, F. Lopez-Gallego, L. Salassa, Chem. Sci. 2017, 8, 4619–4625;
- 18bM.-Y. Yang, C.-J. Chang, L.-Y. Chen, J. Photochem. Photobiol. B 2017, 173, 325–332;
- 18cJ. Gurruchaga-Pereda, V. Martínez-Martínez, E. Rezabal, X. Lopez, C. Garino, F. Mancin, A. L. Cortajarena, L. Salassa, ACS Catal. 2020, 10, 187–196.
- 19
- 19aA. A. Karande, L. Sridhar, K. S. Gopinath, P. R. Adiga, Int. J. Cancer 2001, 95, 277–281;
10.1002/1097-0215(20010920)95:5<277::AID-IJC1047>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 19bX. R. Jiang, X. Y. Yu, J. H. Fan, L. Guo, C. Zhu, W. Jiang, S. H. Lu, Cancer Lett. 2014, 353, 78–86;
- 19cL. Bartmann, D. Schumacher, S. von Stillfried, M. Sternkopf, S. Alampour-Rajabi, M. van Zandvoort, F. Kiessling, Z. Wu, Front. Pharmacol. 2019, 10, 79;
- 19dM. Darguzyte, N. Drude, T. Lammers, F. Kiessling, Cancers 2020, 12, 295.
- 20M. Ishizuka, F. Abe, Y. Sano, K. Takahashi, K. Inoue, M. Nakajima, T. Kohda, N. Komatsu, S. Ogura, T. Tanaka, Int. Immunopharmacol. 2011, 11, 358–365.