Asymmetric Construction of an Aryl-Alkene Axis by Palladium-Catalyzed Suzuki–Miyaura Coupling Reaction
Dr. Sheng-Qi Qiu
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorYu Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Xiang-Jun Peng
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, 341000 China
Search for more papers by this authorDr. Shi-Jiang He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Jun Kee Cheng
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Yong-Bin Wang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Shao-Hua Xiang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorProf. Dr. Jun Song
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peiyuan Yu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junmin Zhang
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bin Tan
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Sheng-Qi Qiu
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorYu Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Xiang-Jun Peng
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, 341000 China
Search for more papers by this authorDr. Shi-Jiang He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Jun Kee Cheng
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Yong-Bin Wang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorDr. Shao-Hua Xiang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorProf. Dr. Jun Song
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peiyuan Yu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junmin Zhang
International Joint Research Center for Molecular Science, College of Chemistry and Environmental Engineering and College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bin Tan
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorGraphical Abstract
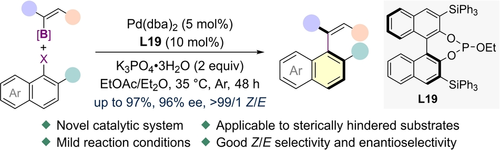
The construction of axially chiral acyclic aryl-alkene skeletons via classic Suzuki–Miyaura reaction has been challenging compared to the biaryls. Rational optimization established an enabling 3,3′-triphenylsilyl-substituted phosphite ligand for asymmetric coupling of hindered aryl halides and vinyl boronates under mild conditions, affording the acyclic aryl-alkenes in good yield, atroposelectivity and E/Z selectivity.
Abstract
The application of Suzuki–Miyaura coupling reaction to forge the atropisomeric biaryls has seen remarkable progress but exploration of this chemistry to directly forge chiral C(aryl)-C(alkene) axis is underdeveloped. The replacement of arene substrates by alkenes intensifies the challenges in terms of reactivity, configurational atropostability of product and selectivity control. By meticulous ligand design and fine-tuning of reaction parameters, we identified a highly active 3,3′-triphenylsilyl-substituted phosphite ligand to realize arene-alkene Suzuki–Miyaura coupling of hindered aryl halides and vinyl boronates under very mild conditions. The axially chiral acyclic aryl-alkenes were generated in commendable efficiency, enantioselectivity and E/Z selectivity.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202211211-sup-0001-4-cifreport(1).pdf86.9 KB | Supporting Information |
| anie202211211-sup-0001-misc_information.pdf11.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193–3207;
- 1bJ. Clayden, W. J. Moran, P. J. Edwards, S. R. LaPlante, Angew. Chem. Int. Ed. 2009, 48, 6398–6401; Angew. Chem. 2009, 121, 6516–6520;
- 1cG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563–639;
- 1dJ. E. Smyth, N. M. Butler, P. A. Keller, Nat. Prod. Rep. 2015, 32, 1562–1583.
- 2
- 2aY. Chen, S. Yekta, A. K. Yudin, Chem. Rev. 2003, 103, 3155–3212;
- 2bP. Kočovský, Š. Vyskočil, M. Smrčina, Chem. Rev. 2003, 103, 3213–3246;
- 2cT. Akiyama, Chem. Rev. 2007, 107, 5744–5758;
- 2d Privileged Chiral Ligands and Catalysts (Ed.: Q.-L. Zhou), Wiley-VCH, Weinheim, 2011;
10.1002/9783527635207 Google Scholar
- 2eD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047–9153;
- 2f Atropisomerism and Axial Chirality (Ed.: J. M. Lassaletta), World Scientific Publishing, Singapore, 2019;
10.1142/q0192 Google Scholar
- 2g Axially Chiral Compounds: Asymmetric Synthesis and Applications (Ed.: B. Tan), Wiley-VCH, Weinheim, 2021.
10.1002/9783527825172 Google Scholar
- 3
- 3aR. Adams, M. W. Miller, J. Am. Chem. Soc. 1940, 62, 53–56;
- 3bT. Kawabata, K. Yahiro, K. Fuji, J. Am. Chem. Soc. 1991, 113, 9694–9696;
- 3cJ. Feng, Z. Gu, SynOpen 2021, 5, 68–85;
- 3dS. Wu, S.-H. Xiang, J. K. Cheng, B. Tan, Tetrahedron Chem 2022, 1, 100009.
10.1016/j.tchem.2022.100009 Google Scholar
- 4
- 4aJ. Feng, B. Li, Y. He, Z. Gu, Angew. Chem. Int. Ed. 2016, 55, 2186–2190; Angew. Chem. 2016, 128, 2226–2230;
- 4bH. Wu, Z. S. Han, B. Qu, D. Wang, Y. Zhang, Y. Xu, N. Grinberg, H. Lee, J. J. Song, F. Roschangar, G. Wang, C. H. Senanayake, Adv. Synth. Catal. 2017, 359, 3927–3933;
- 4cC. Pan, Z. Zhu, M. Zhang, Z. Gu, Angew. Chem. Int. Ed. 2017, 56, 4777–4781; Angew. Chem. 2017, 129, 4855–4859.
- 5S.-C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye, B. Tan, Nat. Commun. 2017, 8, 15238.
- 6
- 6aD. Li, Y. Tan, L. Peng, S. Li, N. Zhang, Y. Liu, H. Yan, Org. Lett. 2018, 20, 4959–4963;
- 6bS. Li, D. Xu, F. Hu, D. Li, W. Qin, H. Yan, Org. Lett. 2018, 20, 7665–7669;
- 6cS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056–7060;
- 6dY. Tan, S. Jia, F. Hu, Y. Liu, L. Peng, D. Li, H. Yan, J. Am. Chem. Soc. 2018, 140, 16893–16898;
- 6eA. Huang, L. Zhang, D. Li, Y. Liu, H. Yan, W. Li, Org. Lett. 2019, 21, 95–99;
- 6fQ.-Z. Li, P.-F. Lian, F.-X. Tan, G.-D. Zhu, C. Chen, Y. Hao, W. Jiang, X.-H. Wang, J. Zhou, S.-Y. Zhang, Org. Lett. 2020, 22, 2448–2453;
- 6gY.-B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang, S.-H. Luo, Q.-S. Gu, K. N. Houk, B. Tan, Nat. Catal. 2019, 2, 504–513;
- 6hJ.-L. Yan, R. Maiti, S.-C. Ren, W. Tian, T. Li, J. Xu, B. Mondal, Z. Jin, Y. R. Chi, Nat. Commun. 2022, 13, 84.
- 7
- 7aY.-B. Wang, Q.-H. Wu, Z.-P. Zhou, S.-H. Xiang, Y. Cui, P. Yu, B. Tan, Angew. Chem. Int. Ed. 2019, 58, 13443–13447; Angew. Chem. 2019, 131, 13577–13581;
- 7bC. Ma, F.-T. Sheng, H.-Q. Wang, S. Deng, Y.-C. Zhang, Y. Jiao, W. Tan, F. Shi, J. Am. Chem. Soc. 2020, 142, 15686–15696;
- 7cP. Kumar, R. P. Shirke, S. Yadav, S. S. V. Ramasastry, Org. Lett. 2021, 23, 4909–4914;
- 7dS. Wu, S.-H. Xiang, S. Li, W.-Y. Ding, L. Zhang, P.-Y. Jiang, Z.-A. Zhou, B. Tan, Nat. Catal. 2021, 4, 692–702;
- 7eR. Mi, H. Chen, X. Zhou, N. Li, D. Ji, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2022, 61, e202111860; Angew. Chem. 2022, 134, e202111860.
- 8
- 8aL. Jin, Q.-J. Yao, P.-P. Xie, Y. Li, B.-B. Zhan, Y.-Q. Han, X. Hong, B.-F. Shi, Chem 2020, 6, 497–511;
- 8bH. Song, Y. Li, Q.-J. Yao, L. Jin, L. Liu, Y.-H. Liu, B.-F. Shi, Angew. Chem. Int. Ed. 2020, 59, 6576–6580; Angew. Chem. 2020, 132, 6638–6642;
- 8cL. Jin, P. Zhang, Y. Li, X. Yu, B.-F. Shi, J. Am. Chem. Soc. 2021, 143, 12335–12344;
- 8dC. Yang, T.-R. Wu, Y. Li, B.-B. Wu, R.-X. Jin, D.-D. Hu, Y.-B. Li, K.-J. Bian, X.-S. Wang, Chem. Sci. 2021, 12, 3726–3732.
- 9J. Wang, X. Qi, X.-L. Min, W. Yi, P. Liu, Y. He, J. Am. Chem. Soc. 2021, 143, 10686–10694.
- 10S.-J. Liu, Z.-H. Chen, J.-Y. Chen, S.-F. Ni, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202112226; Angew. Chem. 2022, 134, e202112226.
- 11
- 11aG. Liao, T. Zhou, Q.-J. Yao, B.-F. Shi, Chem. Commun. 2019, 55, 8514–8523;
- 11bG. Liao, T. Zhang, Z.-K. Lin, B.-F. Shi, Angew. Chem. Int. Ed. 2020, 59, 19773–19786; Angew. Chem. 2020, 132, 19941–19954;
- 11cM. I. Lapuh, S. Mazeh, T. Besset, ACS Catal. 2020, 10, 12898–12919.
- 12
- 12aA. P. Lightfoot, S. J. R. Twiddle, A. Whiting, Synlett 2005, 529–531;
- 12bR. R. Carter, J. K. Wyatt, Tetrahedron Lett. 2006, 47, 6091–6094;
- 12cG. A. Molander, T. Fumagalli, J. Org. Chem. 2006, 71, 5743–5747;
- 12dG. A. Molander, A. R. Brown, J. Org. Chem. 2006, 71, 9681–9686;
- 12eM. D. Brooker, S. M. Cooper Jr, D. R. Hodges, R. R. Carter, J. K. Wyatt, Tetrahedron Lett. 2010, 51, 6748–6752;
- 12fL. Henderson, D. W. Knight, P. Rutkowski, A. C. Williams, Tetrahedron Lett. 2012, 53, 4654–4656.
- 13
- 13aG.-P. Lu, K. R. Voigtritter, C. Cai, B. H. Lipshutz, J. Org. Chem. 2012, 77, 3700–3703;
- 13bB. X. Li, D. N. Le, K. A. Mack, A. McClory, N.-K. Lim, T. Cravillion, S. Savage, C. Han D B Collum, H. Zhang, F. Gosselin, J. Am. Chem. Soc. 2017, 139, 10777–10783;
- 13cH. H. Rau, N. S. Werner, Bioorg. Med. Chem. Lett. 2018, 28, 2693–2696;
- 13dA. Feceu, L. E. Sangster, D. B. C. Martin, Org. Lett. 2018, 20, 3151–3155;
- 13eN. K. Chehal, P. H. M. Budzelaar, P. G. Hultin, Org. Biomol. Chem. 2018, 16, 1134–1143.
- 14
- 14aA. Bermejo, A. Ros, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2008, 130, 15798–15799;
- 14bG. Xu, Q. Zhao, W. Tang, Chin. J. Org. Chem. 2014, 34, 1919–1940;
- 14cG. Xu, W. Fu, G. Liu, C. H. Senanayake, W. Tang, J. Am. Chem. Soc. 2014, 136, 570–573;
- 14dJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418–3430;
- 14eD. Zhang, Q. Wang, Coord. Chem. Rev. 2015, 286, 1–16;
- 14fP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131–190;
- 14gN. D. Patel, J. D. Sieber, S. Tcyrulnikov, B. J. Simmons, D. Rivalti, K. Duvvuri, Y. Zhang, D. A. Gao, K. R. Fandrick, N. Haddad, K. S. Lao, H. P. R. Mangunuru, S. Biswas, B. Qu, N. Grinberg, S. Pennino, H. Lee, J. J. Song, B. F. Gupton, N. K. Garg, M. C. Kozlowski, C. H. Senanayake, ACS Catal. 2018, 8, 10190–10209;
- 14hD. Shen, Y. Xu, S.-L. Shi, J. Am. Chem. Soc. 2019, 141, 14938–14945;
- 14iH. Yang, J. Sun, W. Gu, W. Tang, J. Am. Chem. Soc. 2020, 142, 8036–8043.
- 15
- 15aJ. Yin, M. P. Rainka, X.-X. Zhang, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1162–1163;
- 15bT. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 4685–4696;
- 15cW. Tang, A. G. Capacci, X. Wei, W. Li, A. White, N. D. Patel, J. Savoie, J. J. Gao, S. Rodriguez, B. Qu, N. Haddad, B. Z. Lu, D. Krishnamurthy, N. K. Yee, C. H. Senanayake, Angew. Chem. Int. Ed. 2010, 49, 5879–5883; Angew. Chem. 2010, 122, 6015–6019;
- 15dJ. Sun, W. Gu, H. Yang, W. Tang, Chem. Sci. 2021, 12, 10313–10320.
- 16
- 16aY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534–547;
- 16bB.-C. Da, S.-H. Xiang, S. Li, B. Tan, Chin. J. Chem. 2021, 39, 1787–1796;
- 16cJ. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902.
- 17Deposition Number 2132529 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.