Regio- and Diastereoselective Formal [2+2] Cycloaddition of Allenes with Amino-Functionalized Alkenes by Rare-Earth-Catalyzed C(sp2)−H Activation
Wenxuan Xu
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Search for more papers by this authorCorresponding Author
Dr. Xuefeng Cong
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Kun An
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Shao-Jie Lou
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorZhenghua Li
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Masayoshi Nishiura
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorProf. Dr. Tetsuro Murahashi
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaomin Hou
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorWenxuan Xu
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Search for more papers by this authorCorresponding Author
Dr. Xuefeng Cong
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Kun An
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Shao-Jie Lou
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorZhenghua Li
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorDr. Masayoshi Nishiura
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorProf. Dr. Tetsuro Murahashi
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaomin Hou
Advanced Catalysis Research Group, RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152–8552 Japan
Organometallic Chemistry Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351–0198 Japan
Search for more papers by this authorGraphical Abstract
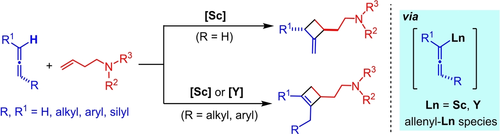
Half-sandwich rare-earth catalysts serve as a unique platform for the regio- and diastereoselective formal [2+2] cycloaddition of a wide range of allenes with amino-functionalized alkenes via allene C(sp2)−H activation, affording a new family of cyclobutane and cyclobutene derivatives which were difficult to access previously.
Abstract
The [2+2] cycloaddition of allenes with alkenes is of much interest and importance as a straightforward route for the construction of four-membered carbocycles but has remained much underexplored to date. Herein we report for the first time the intermolecular regio- and diastereoselective formal [2+2] cycloaddition of a wide range of allenes with amino-functionalized alkenes by half-sandwich rare-earth catalysts. The reaction proceeded through an allene C(sp2)−H activation mechanism initiated by the site-selective deprotonation of the allene unit by a rare-earth metal alkyl species followed by alkene insertion into the resulting metal-allenyl bond and the subsequent intramolecular cycloaddition to an allene C=C bond. This protocol offers a unique route for the synthesis of a new family of cyclobutane and cyclobutene derivatives which were difficult to access previously.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202210624-sup-0001-misc_information.pdf29.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. S. S. Damsté, M. Strous, W. I. C. Rijpstra, E. C. Hopmans, J. A. Geenevasen, A. C. Van Duin, L. A. Van Niftrik, M. S. Jetten, Nature 2002, 419, 708;
- 1bE. M. Carreira, T. C. Fessard, Chem. Rev. 2014, 114, 8257.
- 2
- 2aJ. C. Namyslo, D. E. Kaufmann, Chem. Rev. 2003, 103, 1485;
- 2bE. Lee-Ruff, G. Mladenova, Chem. Rev. 2003, 103, 1449;
- 2cT. Seiser, T. Saget, D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 7740; Angew. Chem. 2011, 123, 7884;
- 2dG. Fumagalli, S. Stanton, J. F. Bower, Chem. Rev. 2017, 117, 9404.
- 3Selected reviews on the synthesis of cyclobutanes by the [2+2] cycloaddition of alkenes:
- 3aY. Xu, M. L. Conner, M. K. Brown, Angew. Chem. Int. Ed. 2015, 54, 11918; Angew. Chem. 2015, 127, 12086;
- 3bS. Poplata, A. Tröster, Y. Q. Zou, T. Bach, Chem. Rev. 2016, 116, 9748;
- 3cM. D. Kärkäs Jr., J. A. Porco, C. R. J. Stephenson, Chem. Rev. 2016, 116, 9683;
- 3dT. P. Yoon, Acc. Chem. Res. 2016, 49, 2307;
- 3eP. J. Chirik, Angew. Chem. Int. Ed. 2017, 56, 5170; Angew. Chem. 2017, 129, 5252;
- 3fD. P. Schwinger, T. Bach, Acc. Chem. Res. 2020, 53, 1933.
- 4For examples of the synthesis of cyclobutenes by the [2+2] cycloaddition of alkenes with alkynes, see:
- 4aV. López-Carrillo, A. M. Echavarren, J. Am. Chem. Soc. 2010, 132, 9292;
- 4bM. E. De Orbe, L. Amenós, M. S. Kirillova, Y. Wang, V. López-Carrillo, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2017, 139, 10302;
- 4cC. García-Morales, B. Ranieri, I. Escofet, L. López-Suarez, C. Obradors, A. I. Konovalov, A. M. Echavarren, J. Am. Chem. Soc. 2017, 139, 13628;
- 4dL. Shen, K. Zhao, K. Doitomi, R. Ganguly, Y. X. Li, Z. L. Shen, H. Hirao, T. P. Loh, J. Am. Chem. Soc. 2017, 139, 13570;
- 4eY. B. Bai, Z. Luo, Y. Wang, J. M. Gao, L. Zhang, J. Am. Chem. Soc. 2018, 140, 5860;
- 4fM. M. Parsutkar, V. V. Pagar, T. V. RajanBabu, J. Am. Chem. Soc. 2019, 141, 15367;
- 4gS. Ha, Y. Lee, Y. Kwak, A. Mishra, E. Yu, B. Ryou, C. M. Park, Nat. Commun. 2020, 11, 2509.
- 5Selected reviews on the intramolecular [2+2] cycloaddition of allenes with alkenes:
- 5aS. Ma, Chem. Rev. 2005, 105, 2829;
- 5bB. Alcaide, P. Almendros, C. Aragoncillo, Chem. Soc. Rev. 2010, 39, 783;
- 5cA. Brandi, S. Cicchi, F. M. Cordero, A. Goti, Chem. Rev. 2014, 114, 7317;
- 5dM. R. Fructos, A. Prieto, Tetrahedron 2016, 72, 355.
- 6Selected examples of intramolecular [2+2] cycloaddition of allenes with alkenes:
- 6aM. R. Luzung, P. Mauleón, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 12402;
- 6bH. Teller, S. Flügge, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2010, 49, 1949; Angew. Chem. 2010, 122, 1993;
- 6cM. Gulías, A. Collado, B. Trillo, F. López, E. Oñate, M. A. Esteruelas, J. L. Mascareñas, J. Am. Chem. Soc. 2011, 133, 7660;
- 6dA. Z. González, D. Benitez, E. Tkatchouk, W. A. Goddard III, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 5500;
- 6eN. N. Noucti, E. J. Alexanian, Angew. Chem. Int. Ed. 2015, 54, 5447; Angew. Chem. 2015, 127, 5537;
- 6fT. Nada, Y. Yoneshige, Y. Ii, T. Matsumoto, H. Fujioka, S. Shuto, M. Arisawa, ACS Catal. 2016, 6, 3168.
- 7For examples of thermal and photochemical intermolecular [2+2] cycloaddition of allenes with polarized alkenes, see:
- 7aD. J. Pasto, S. E. Warren, J. Am. Chem. Soc. 1982, 104, 3670;
- 7bD. J. Pasto, S. H. Yang, J. Am. Chem. Soc. 1984, 106, 152;
- 7cD. J. Pasto, S. H. Yang, J. Org. Chem. 1986, 51, 1676;
- 7dK. Maruyama, H. Imahori, J. Org. Chem. 1989, 54, 2692;
- 7eB. Lohmeyer, K. Schmidt, P. Margaretha, Helv. Chim. Acta 2006, 89, 854.
- 8For examples of Lewis acid-catalyzed intermolecular [2+2] cycloaddition of polarized allenes with alkenes, see:
- 8aS. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio, J. M. González, Angew. Chem. Int. Ed. 2012, 51, 11552; Angew. Chem. 2012, 124, 11720;
- 8bS. Suárez-Pantiga, C. Hernández-Díaz, M. Piedrafita, E. Rubio, J. M. González, Adv. Synth. Catal. 2012, 354, 1651;
- 8cH. Faustino, P. Bernal, L. Castedo, F. López, J. L. Mascareñas, Adv. Synth. Catal. 2012, 354, 1658;
- 8dJ. Francos, F. Grande-Carmona, H. Faustino, J. Iglesias-Sigüenza, E. Díez, I. Alonso, R. Fernández, J. M. Lassaletta, F. López, J. L. Mascareñas, J. Am. Chem. Soc. 2012, 134, 14322;
- 8eH. Faustino, I. Alonso, J. L. Mascareñas, F. López, Angew. Chem. Int. Ed. 2013, 52, 6526; Angew. Chem. 2013, 125, 6654;
- 8fY. Wang, P. Zhang, Y. Liu, F. Xia, J. Zhang, Chem. Sci. 2015, 6, 5564;
- 8gM. L. Conner, Y. Xu, M. K. Brown, J. Am. Chem. Soc. 2015, 137, 3482;
- 8hJ. M. Wahl, M. L. Conner, M. K. Brown, Angew. Chem. Int. Ed. 2018, 57, 4647; Angew. Chem. 2018, 130, 4737;
- 8iX. Zhong, J. Tan, J. Qiao, Y. Zhou, C. Lv, Z. Su, S. Dong, X. Feng, Chem. Sci. 2021, 12, 9991.
- 9For examples of stoichiometric deprotonation of allenes by bases, see:
- 9aA. Maercker, J. Fischenich, Tetrahedron 1995, 51, 10209;
- 9bH. Xiong, R. P. Hsung, L. L. Wei, C. R. Berry, J. A. Mulder, B. Stockwell, Org. Lett. 2000, 2, 2869;
- 9cJ. Zhao, Y. Liu, S. Ma, Org. Lett. 2008, 10, 1521;
- 9dN. Alouane, K. Bentayeb, E. Vrancken, H. Gérard, P. Mangeney, Chem. Eur. J. 2009, 15, 45.
- 10For formal catalytic C−H functionalization of allenes through the Heck-type cross-coupling, see:
- 10aC. Fu, S. Ma, Org. Lett. 2005, 7, 1605;
- 10bR. Zeng, S. Wu, C. Fu, S. Ma, J. Am. Chem. Soc. 2013, 135, 18284;
- 10cS. Nakanowatari, L. Ackermann, Chem. Eur. J. 2015, 21, 16246;
- 10dG. Wang, X. Liu, Y. Chen, J. Yang, J. Li, L. Lin, X. Feng, ACS Catal. 2016, 6, 2482;
- 10eY. Wang, S. G. Scrivener, X. D. Zuo, R. Wang, P. N. Palermo, E. Murphy, A. C. Durham, Y. M. Wang, J. Am. Chem. Soc. 2021, 143, 14998.
- 11The first true catalytic allene C(sp2)−H functionalization with an alkene was recently reported. See: B. S. Schreib, M. Son, F. A. Aouane, M. H. Baik, E. M. Carreira, J. Am. Chem. Soc. 2021, 143, 21705.
- 12Selected reviews:
- 12aM. Nishiura, Z. Hou, Nat. Chem. 2010, 2, 257;
- 12bM. Nishiura, F. Guo, Z. Hou, Acc. Chem. Res. 2015, 48, 2209;
- 12cP. L. Arnold, M. W. McMullon, J. Rieb, F. E. Kühn, Angew. Chem. Int. Ed. 2015, 54, 82; Angew. Chem. 2015, 127, 84;
- 12dY. Yang, M. Nishiura, H. Wang, Z. Hou, Coord. Chem. Rev. 2018, 376, 506;
- 12eP. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz, L. Ackermann, Chem. Rev. 2019, 119, 2192.
- 13Selected examples of rare-earth-catalyzed C−H activation and transformation:
- 13aB. T. Guan, Z. Hou, J. Am. Chem. Soc. 2011, 133, 18086;
- 13bJ. Oyamada, Z. Hou, Angew. Chem. Int. Ed. 2012, 51, 12828; Angew. Chem. 2012, 124, 13000;
- 13cB. T. Guan, B. Wang, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2013, 52, 4418; Angew. Chem. 2013, 125, 4514;
- 13dG. Song, W. W. N. O, Z. Hou, J. Am. Chem. Soc. 2014, 136, 12209;
- 13eG. Song, B. Wang, M. Nishiura, Z. Hou, Chem. Eur. J. 2015, 21, 8394;
- 13fG. Song, G. Luo, J. Oyamada, Y. Luo, Z. Hou, Chem. Sci. 2016, 7, 5265;
- 13gA. E. Nako, J. Oyamada, M. Nishiura, Z. Hou, Chem. Sci. 2016, 7, 6429;
- 13hY. Luo, H. Teng, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2017, 56, 9207; Angew. Chem. 2017, 129, 9335;
- 13iY. Luo, Y. Ma, Z. Hou, J. Am. Chem. Soc. 2018, 140, 114;
- 13jS. J. Lou, Z. Mo, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2020, 142, 1200;
- 13kX. Cong, G. Zhan, Z. Mo, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2020, 142, 5531;
- 13lS. J. Lou, Q. Zhuo, M. Nishiura, G. Luo, Z. Hou, J. Am. Chem. Soc. 2021, 143, 2470;
- 13mS. J. Lou, G. Luo, S. Yamaguchi, K. An, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2021, 143, 20462;
- 13nX. Cong, Q. Zhuo, N. Hao, Z. Mo, G. Zhan, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2022, 61, e202115996; Angew. Chem. 2022, 134, e202115996.
- 14Selected examples of polymerization by half-sandwich rare-earth catalysts:
- 14aX. Shi, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2016, 138, 6147;
- 14bX. Shi, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2016, 55, 14812; Angew. Chem. 2016, 128, 15032;
- 14cC. Wang, G. Luo, M. Nishiura, G. Song, A. Yamamoto, Y. Luo, Z. Hou, Sci. Adv. 2017, 3, e1701011;
- 14dH. Wang, Y. Yang, M. Nishiura, Y. Higaki, A. Takahara, Z. Hou, J. Am. Chem. Soc. 2019, 141, 3249;
- 14eH. Wang, Y. Zhao, M. Nishiura, Y. Yang, G. Luo, Y. Luo, Z. Hou, J. Am. Chem. Soc. 2019, 141, 12624;
- 14fH. Wang, X. Wu, Y. Yang, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2020, 59, 7173; Angew. Chem. 2020, 132, 7240;
- 14gY. Yang, H. Wang, L. Huang, M. Nishiura, Y. Higaki, Z. Hou, Angew. Chem. Int. Ed. 2021, 60, 26192; Angew. Chem. 2021, 133, 26396.
- 15X. Li, M. Nishiura, K. Mori, T. Mashiko, Z. Hou, Chem. Commun. 2007, 4137.
- 16For examples of allene polymerization by rare-earth catalysts, see: Y. Zhong, I. Douair, T. Wang, C. Wu, L. Maron, D. Cui, Angew. Chem. Int. Ed. 2020, 59, 4947; Angew. Chem. 2020, 132, 4977.
- 17The analogous amino-functionalized 1-propylene, 1-pentene and 1-hexene were not effective for the annulation with 1 a (only the polymerization of 1 a was observed). In the case of ether- and thioether-functionalized alkenes, the polymerization of alkenes took place and the annulation with 1 a was not observed. For the influences of heteroatoms and the spacer length between the heteroatom and the C=C bond on the polymerization of heteroatom-functionalized alkenes by rare-earth catalysts, see refs. [14c–g].
- 18T. Shima, M. Nishiura, Z. Hou, Organometallics 2011, 30, 2513.
- 19Ionic radius with coordination number of six: Sc3+, 0.745 Å; Y3+, 0.900 Å; Lu3+, 0.860 Å; Er3+, 0.890 Å. See: R. D. Shannon, Acta Crystallogr. Sect. A 1976, 32, 751.
- 20Y. Luo, J. Baldamus, Z. Hou, J. Am. Chem. Soc. 2004, 126, 13910.
- 21For examples of heteroatom-assisted olefin polymerization by rare-earth catalysts, see refs. [14c–g]. The lack of alkene polymerization in the case of alkenes bearing N-benzyl groups might be due to the relatively larger steric hindrance around the N atom.
- 22W. J. Jang, S. M. Song, J. H. Moon, J. Y. Lee, J. Yun, J. Am. Chem. Soc. 2017, 139, 13660.
- 23S. Da Silva Pinto, S. G. Davies, A. M. Fletcher, P. M. Roberts, J. E. Thomson, J. Org. Chem. 2018, 83, 9939.
- 24
- 24aR. A. Baillie, P. Legzdins, Acc. Chem. Res. 2014, 47, 330;
- 24bT. Bai, S. Ma, G. Jia, Coord. Chem. Rev. 2009, 253, 423.
- 25
- 25aS. Standfuss, E. Abinet, T. P. Spaniol, J. Okuda, Chem. Commun. 2011, 47, 11441;
- 25bN. Yu, M. Nishiura, X. Li, Z. Xi, Z. Hou, Chem. Asian J. 2008, 3, 1406.
- 26For examples of metal-phenyl interaction in similar cases, see:
- 26aY. Luo, Y. Luo, J. Qu, Z. Hou, Organometallics 2011, 30, 2908;
- 26bX. Kang, A. Yamamoto, M. Nishiura, Y. Luo, Z. Hou, Organometallics 2015, 34, 5540;
- 26cP. Wang, G. Luo, J. Yang, X. Cong, Z. Hou, Y. Luo, J. Org. Chem. 2021, 86, 4236. See also ref. [13k].
- 27For a possible reaction mechanism of the Sc-1-catalyzed reaction of 4 l with 2 a, see the Supporting Information.