Yttrium-Catalyzed ortho-Selective C−H Borylation of Pyridines with Pinacolborane
Yuncong Luo
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorShengjie Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xin Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorYuncong Luo
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorShengjie Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xin Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
Search for more papers by this authorGraphical Abstract
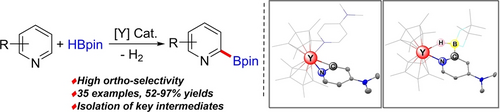
The ortho-selective C−H borylation of a wide range of pyridines using pinacolborane was achieved through yttrium catalysis. Notably, the possible hydroboration side reaction was effectively suppressed by using the proper ligand/metal combination. The resultant 2-pyridyl boronates were subjected to further transformations such as a Suzuki–Miyaura coupling or the Chan–Lam amination.
Abstract
This work reports a site-selective C−H borylation of pyridines at the ortho-position with pinacolborane enabled by an yttrocene catalyst. The reaction provides a new family of 2-pyridyl boronates with a broad substrate scope and high atom efficiency. The resultant boronates were able to undergo a variety of transformations, e.g., oxidation, Suzuki–Miyaura coupling, Chan–Lam amination and etherification. Catalytic intermediates, including ortho-C−H metalated and borylated complexes, were isolated from stoichiometric experiments and confirmed by single-crystal X-ray diffraction.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202117750-sup-0001-Checkcifs.pdf382.1 KB | Supporting Information |
| anie202117750-sup-0001-complex_10.cif1.3 MB | Supporting Information |
| anie202117750-sup-0001-complex_11.cif1.2 MB | Supporting Information |
| anie202117750-sup-0001-compound_14.cif485.2 KB | Supporting Information |
| anie202117750-sup-0001-compound_9c.cif2.8 MB | Supporting Information |
| anie202117750-sup-0001-compound_9n.cif2.5 MB | Supporting Information |
| anie202117750-sup-0001-compound_9t.cif472.5 KB | Supporting Information |
| anie202117750-sup-0001-misc_information.pdf7.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Furuya, H. M. Kaiser, T. Ritter, Angew. Chem. Int. Ed. 2008, 47, 5993–5996; Angew. Chem. 2008, 120, 6082–6085;
- 1bA. Suzuki, Angew. Chem. Int. Ed. 2011, 50, 6722–6737; Angew. Chem. 2011, 123, 6854–6869;
- 1cA. Bonet, M. Odachowski, D. Leonori, S. Essafi, V. K. Aggarwal, Nat. Chem. 2014, 6, 584–589;
- 1dM. J. West, J. W. B. Fyfe, J. C. Vantourout, A. J. B. Watson, Chem. Rev. 2019, 119, 12491–12523.
- 2 Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials (Ed.: D. G. Hall), Wiley-VCH, Weinheim, 2011.
- 3Selected reviews for C−H borylation:
- 3aI. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931;
- 3bJ. F. Hartwig, Chem. Soc. Rev. 2011, 40, 1992–2002;
- 3cJ. F. Hartwig, Acc. Chem. Res. 2012, 45, 864–873;
- 3dA. Ros, R. Fernández, J. M. Lassaletta, Chem. Soc. Rev. 2014, 43, 3229–3243;
- 3eL. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao, P. Li, Tetrahedron 2017, 73, 7123–7157;
- 3fC. Haldar, M. E. Hoque, R. Bisht, B. Chattopadhyay, Tetrahedron Lett. 2018, 59, 1269–1277;
- 3gM. E. Hoque, M. M. M. Hassan, B. Chattopadhyay, J. Am. Chem. Soc. 2021, 143, 5022–5037;
- 3hJ. S. Wright, P. J. H. Scott, P. G. Steel, Angew. Chem. Int. Ed. 2021, 60, 2796–2821; Angew. Chem. 2021, 133, 2830–2856.
- 4Selected examples for metal-catalyzed ortho-C−H borylation:
- 4aT. A. Boebel, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 7534–7535;
- 4bS. Kawamorita, H. Ohmiya, K. Hara, A. Fukuoka, M. Sawamura, J. Am. Chem. Soc. 2009, 131, 5058–5059;
- 4cB. Ghaffari, S. M. Preshlock, D. L. Plattner, R. J. Staples, P. E. Maligres, S. W. Krska, R. E. Maleczka, M. R. Smith, J. Am. Chem. Soc. 2014, 136, 14345–14348;
- 4dG. Wang, L. Liu, H. Wang, Y.-S. Ding, J. Zhou, S. Mao, P. Li, J. Am. Chem. Soc. 2017, 139, 91–94;
- 4eS.-T. Bai, C. B. Bheeter, J. N. H. Reek, Angew. Chem. Int. Ed. 2019, 58, 13039–13043; Angew. Chem. 2019, 131, 13173–13177;
- 4fJ. Thongpaen, R. Manguin, V. Dorcet, T. Vives, C. Duhayon, M. Mauduit, O. Baslé, Angew. Chem. Int. Ed. 2019, 58, 15244–15248; Angew. Chem. 2019, 131, 15388–15392;
- 4gR. Bisht, J. Chaturvedi, G. Pandey, B. Chattopadhyay, Org. Lett. 2019, 21, 6476–6480;
- 4hM. M. M. Hassan, M. E. Hoque, S. Dey, S. Guria, B. Roy, B. Chattopadhyay, Synthesis 2021, 53, 3333–3342.
- 5
- 5aA. R. Katritzky, C. W. Rees, E. F. V. Scriven, Comprehensive Heterocyclic Chemistry II, 1st ed., Pergamon, Oxford, 1996;
- 5bJ. P. Michael, Nat. Prod. Rep. 2005, 22, 627–646;
- 5cM. D. Hill, Chem. Eur. J. 2010, 16, 12052–12062.
- 6
- 6aJ. Takagi, K. Sato, J. F. Hartwig, T. Ishiyama, N. Miyaura, Tetrahedron Lett. 2002, 43, 5649–5651;
- 6bI. A. I. Mkhalid, D. N. Coventry, D. Albesa-Jove, A. S. Batsanov, J. A. K. Howard, R. N. Perutz, T. B. Marder, Angew. Chem. Int. Ed. 2006, 45, 489–491; Angew. Chem. 2006, 118, 503–505;
- 6cS. A. Sadler, H. Tajuddin, I. A. I. Mkhalid, A. S. Batsanov, D. Albesa-Jove, M. S. Cheung, A. C. Maxwell, L. Shukla, B. Roberts, D. C. Blakemore, Z. Lin, T. B. Marder, P. G. Steel, Org. Biomol. Chem. 2014, 12, 7318–7327;
- 6dJ. V. Obligacion, S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2014, 136, 4133–4136;
- 6eK. Murakami, S. Yamada, T. Kaneda, K. Itami, Chem. Rev. 2017, 117, 9302–9332.
- 7L. Yang, K. Semba, Y. Nakao, Angew. Chem. Int. Ed. 2017, 56, 4853–4857; Angew. Chem. 2017, 129, 4931–4935.
- 8
- 8aL. Yang, N. Uemura, Y. Nakao, J. Am. Chem. Soc. 2019, 141, 7972–7979;
- 8bJ. Trouvé, P. Zardi, S. Al-Shehimy, T. Roisnel, R. Gramage-Doria, Angew. Chem. Int. Ed. 2021, 60, 18006–18013.
- 9
- 9aL.-C. Campeau, S. Rousseaux, K. Fagnou, J. Am. Chem. Soc. 2005, 127, 18020–18021;
- 9bG. R. Dick, E. M. Woerly, M. D. Burke, Angew. Chem. Int. Ed. 2012, 51, 2667–2672; Angew. Chem. 2012, 124, 2721–2726;
- 9cM. A. Larsen, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 4287–4299;
- 9dX. A. F. Cook, A. de Gombert, J. McKnight, L. R. E. Pantaine, M. C. Willis, Angew. Chem. Int. Ed. 2021, 60, 11068–11091.
- 10J. H. Kim, T. Constantin, M. Simonetti, J. Llaveria, N. S. Sheikh, D. Leonori, Nature 2021, 595, 677–683.
- 11P. Xu, X. Xu, Organometallics 2019, 38, 3212–3217.
- 12
- 12aG. Jeske, H. Lauke, H. Mauermann, P. N. Swepston, H. Schumann, T. J. Marks, J. Am. Chem. Soc. 1985, 107, 8091–8103;
- 12bS. G. Minasian, J. D. Rinehart, P. Bazinet, M. Seitz, Acta Crystallogr. Sect. E 2006, 62, m1823-m1824;
- 12cY. Luo, H.-L. Teng, C. Xue, M. Nishiura, Z. Hou, ACS Catal. 2018, 8, 8027–8032.
- 13A lutetium-catalyzed C−H ortho-borylation of azines has been reported during the preparation of this manuscript. See: J. O. Rothbaum, A. Motta, Y. Kratish, T. J. Marks, ChemRxiv. 2021, https://doi.org/10.26434/chemrxiv-2021-38h45. This content is a preprint and has not been peer-reviewed.
- 14A. S. Dudnik, V. L. Weidner, A. Motta, M. Delferro, T. J. Marks, Nat. Chem. 2014, 6, 1100–1107.
- 15
- 15aX. Cong, G. Zhan, Z. Mo, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2020, 142, 5531–5537;
- 15bS.-J. Lou, L. Zhang, Y. Luo, M. Nishiura, G. Luo, Y. Luo, Z. Hou, J. Am. Chem. Soc. 2020, 142, 18128–18137.
- 16C. Xue, Y. Luo, H. Teng, Y. Ma, M. Nishiura, Z. Hou, ACS Catal. 2018, 8, 5017–5022.
- 17
- 17aB.-T. Guan, Z. Hou, J. Am. Chem. Soc. 2011, 133, 18086–18089;
- 17bP. L. Arnold, M. W. McMullon, J. Rieb, F. E. Kühn, Angew. Chem. Int. Ed. 2015, 54, 82–100; Angew. Chem. 2015, 127, 84–103;
- 17cM. Nishiura, F. Guo, Z. Hou, Acc. Chem. Res. 2015, 48, 2209–2220;
- 17dH. Nagae, Y. Shibata, H. Tsurugi, K. Mashima, J. Am. Chem. Soc. 2015, 137, 640–643;
- 17eG. Zhou, G. Luo, X. Kang, Z. Hou, Y. Luo, Organometallics 2018, 37, 2741–2748;
- 17fJ. Su, X. Xu, J. Chin. Soc. Rare Earths. 2021, 39, 171–180.
- 18A. L. Reznichenko, K. C. Hultzsch, Top. Organomet. Chem. Vol. 43, Springer, Berlin, Heidelberg, 2011, pp. 51–114.
- 19
- 19aJ. Oyamada, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2011, 50, 10720–10723; Angew. Chem. 2011, 123, 10908–10911;
- 19bJ. Su, Y. Zhou, X. Xu, Org. Biomol. Chem. 2019, 17, 2013–2019.
- 20B.-J. Deelman, W. M. Stevels, J. H. Teuben, M. T. Lakin, A. L. Spek, Organometallics 1994, 13, 3881–3891.
- 21K. H. den Haan, Y. Wielstra, J. H. Teuben, Organometallics 1987, 6, 2053–2060.
- 22E. Y.-X. Chen, T. J. Marks, Chem. Rev. 2000, 100, 1391–1434.
- 23K. H. den Haan, J. H. Teuben, Recl. Trav. Chim. Pays-Bas 1984, 103, 333–334.
10.1002/recl.19841031107 Google Scholar
- 24
- 24aP. A. Cox, A. G. Leach, A. D. Campbell, G. C. Lloyd-Jones, J. Am. Chem. Soc. 2016, 138, 9145–9157;
- 24bP. A. Cox, M. Reid, A. G. Leach, A. D. Campbell, E. J. King, G. C. Lloyd-Jones, J. Am. Chem. Soc. 2017, 139, 13156–13165.
- 25J. A. G. Williams, Chem. Soc. Rev. 2009, 38, 1783–1801.
- 26C. Kaes, A. Katz, M. W. Hosseini, Chem. Rev. 2000, 100, 3553–3590.
- 27Deposition numbers 2130948 (9 c), 2130949 (9 n), 2130950 (9 t), 2130951 (10), 2130952 (11), 2130953 (14) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.