Bio-adhesive Nanoporous Module: Toward Autonomous Gating
Hyuna Jo
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorDr. Takashi Kitao
Department of Advanced Materials Science, Graduate School of Frontier Sciences and Department of Applied Chemistry, Graduate School of Engineering, The University of Tokyo, Chiba, 227-8561 Japan
Search for more papers by this authorAyumi Kimura
Institute of Engineering Innovation, The University of Tokyo, 2-11-16 Yayoi, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorDr. Yoshimitsu Itoh
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takuzo Aida
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
RIKEN Center for Emergent Matter Science, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Search for more papers by this authorCorresponding Author
Dr. Kou Okuro
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, China
Search for more papers by this authorHyuna Jo
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorDr. Takashi Kitao
Department of Advanced Materials Science, Graduate School of Frontier Sciences and Department of Applied Chemistry, Graduate School of Engineering, The University of Tokyo, Chiba, 227-8561 Japan
Search for more papers by this authorAyumi Kimura
Institute of Engineering Innovation, The University of Tokyo, 2-11-16 Yayoi, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorDr. Yoshimitsu Itoh
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takuzo Aida
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
RIKEN Center for Emergent Matter Science, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Search for more papers by this authorCorresponding Author
Dr. Kou Okuro
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656 Japan
Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, China
Search for more papers by this authorGraphical Abstract
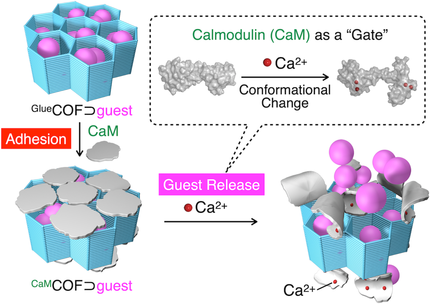
A porous covalent organic framework (GlueCOF) carrying guanidinium ion pendants has been developed that can accommodate guests and bind to biopolymers. The strong bio-adhesive nature of GlueCOF was used to noncovalently bind to calmodulin (CaM), which acted as a gating component for the guest-loaded 1D nanopores (CaMCOF⊃guest). The conformational change of the CaM gate upon selective binding with Ca2+ enabled the release of guest molecules.
Abstract
Here we report a bio-adhesive porous organic module (GlueCOF) composed of hexagonally packed 1D nanopores based on a covalent organic framework. The nanopores are densely decorated with guanidinium ion (Gu+) pendants capable of forming salt bridges with oxyanionic species. GlueCOF strongly adheres to biopolymers through multivalent salt-bridging interactions with their ubiquitous oxyanionic species. By taking advantage of its strong bio-adhesive nature, we succeeded in creating a gate that possibly opens the nanopores through a selective interaction with a reporter chemical and releases guest molecules. We chose calmodulin (CaM) as a gating component that can stably entrap a loaded guest, sulforhodamine B (SRB), within the nanopores (CaMCOF⊃SRB). CaM is known to change its conformation on binding with Ca2+ ions. We confirmed that mixing CaMCOF⊃SRB with Ca2+ resulted in the release of SRB from the nanopores, whereas the use of weakly binding Mg2+ ions resulted in a much slower release of SRB.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017117-sup-0001-misc_information.pdf11.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. E. Uhrich, S. M. Cannizzaro, R. S. Langer, K. M. Shakesheff, Chem. Rev. 1999, 99, 3181–3198;
- 1bI. I. Slowing, J. L. Vivero-Escoto, C. W. Wu, V. S.-Y. Lin, Adv. Drug Delivery Rev. 2008, 60, 1278–1288;
- 1cH. K. Makadia, S. J. Siegel, Polymers 2011, 3, 1377–1397;
- 1dS. Mura, J. Nicolas, P. Couvreur, Nat. Mater. 2013, 12, 991–1003;
- 1eN. Kamaly, B. Yameen, J. Wu, O. C. Farokhzad, Chem. Rev. 2016, 116, 2602–2663.
- 2
- 2aQ. Fu, G. V. R. Rao, L. K. Ista, Y. Wu, B. P. Andrzejewski, L. A. Sklar, T. L. Ward, G. P. López, Adv. Mater. 2003, 15, 1262–1266;
- 2bR. Hernandez, H.-R. Tseng, J. W. Wong, J. F. Stoddart, J. I. Zink, J. Am. Chem. Soc. 2004, 126, 3370–3371;
- 2cY. Zhu, M. Fujiwara, Angew. Chem. Int. Ed. 2007, 46, 2241–2244; Angew. Chem. 2007, 119, 2291–2294;
- 2dJ. Lu, E. Choi, F. Tamanoi, J. I. Zink, Small 2008, 4, 421–426;
- 2eA. F. Moreira, D. R. Dias, I. J. Correia, Microporous Mesoporous Mater. 2016, 236, 141–157;
- 2fC.-A. Cheng, T. Deng, F.-C. Lin, Y. Cai, J. I. Zink, Theranostics 2019, 9, 3341–3364.
- 3S. Zhu, C. Chen, Z. Chen, X. Liu, Y. Li, Y. Shi, D. Zhang, Mater. Chem. Phys. 2011, 126, 357–363.
- 4
- 4aS. Tang, X. Huang, X. Chen, N. Zheng, Adv. Funct. Mater. 2010, 20, 2442–2447;
- 4bM. D. Wang, G. C. Gong, J. Feng, T. Wang, C. D. Ding, B. J. Zhou, W. Jiang, J. J. Fu, ACS Appl. Mater. Interfaces 2016, 8, 23289–23301.
- 5
- 5aJ. W. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. F. Stoddart, O. M. Yaghi, Chem. Sci. 2013, 4, 2858–2864;
- 5bS. Nagata, K. Kokado, K. Sada, Chem. Commun. 2015, 51, 8614–8617;
- 5cL.-L. Tan, N. Song, S. X.-A. Zhang, H. Li, B. Wang, Y.-W. Yang, J. Mater. Chem. B 2016, 4, 135–140;
- 5dX. Meng, B. Gui, D. Yuan, M. Zeller, C. Wang, Sci. Adv. 2016, 2, e1600480.
- 6E. Aznar, M. Oroval, L. Pascual, J. R. Murguía, R. Martínez-Máñez, F. Sancenón, Chem. Rev. 2016, 116, 561–718.
- 7
- 7aV. C. Özalp, T. Schäfer, Chem. Eur. J. 2011, 17, 9893–9896;
- 7bD. He, X. He, K. Wang, J. Cao, Y. Zhao, Adv. Funct. Mater. 2012, 22, 4704–4710.
- 8
- 8aQ. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu, Y. Yan, J. Am. Chem. Soc. 2015, 137, 8352–8355;
- 8bS. Kandambeth, V. Venkatesh, D. B. Shinde, S. Kumari, A. Halder, S. Verma, R. Banerjee, Nat. Commun. 2015, 6, 6786;
- 8cL. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gao, Y. Zhao, Chem. Commun. 2016, 52, 4128–4131;
- 8dD. Liu, W. Zhang, Q. Zeng, S. A. Lei, Chem. Eur. J. 2016, 22, 6768–6773;
- 8eV. S. Vyas, M. Vishwakarma, I. Moudrakovski, F. Haase, G. Savasci, C. Ochsenfeld, J. P. Spatz, B. V. Lotsch, Adv. Mater. 2016, 28, 8749–8754;
- 8fS. Mitra, H. S. Sasmal, T. Kundu, S. Kandambeth, K. Illath, D. D. Diaz, R. Banerjee, J. Am. Chem. Soc. 2017, 139, 4513–4520;
- 8gG. Zhang, X. Li, Q. Liao, Y. Liu, K. Xi, W. Huang, X. Jia, Nat. Commun. 2018, 9, 2785;
- 8hS. Liu, C. Hu, Y. Liu, X. Zhao, M. Pang, J. Lin, Chem. Eur. J. 2019, 25, 4315–4319;
- 8iQ. Guan, L.-L. Zhou, F.-H. Lv, W.-Y. Li, Y.-A. Li, Y.-B. Dong, Angew. Chem. Int. Ed. 2020, 59, 18042–18047; Angew. Chem. 2020, 132, 18198–18203.
- 9M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder, W. R. Dichtel, J. Am. Chem. Soc. 2017, 139, 4999–5002.
- 10
- 10aN. Sakai, S. Matile, J. Am. Chem. Soc. 2003, 125, 14348–14356;
- 10bA. Hennig, G. J. Gabriel, G. N. Tew, S. Matile, J. Am. Chem. Soc. 2008, 130, 10338–10344;
- 10cD. Shukla, C. P. Schneider, B. L. Trout, J. Am. Chem. Soc. 2011, 133, 18713–18718;
- 10dE. I. Geihe, C. B. Cooley, J. R. Simon, M. K. Kiesewetter, J. A. Edward, R. P. Hickerson, R. L. Kaspar, J. L. Hedrick, R. M. Waymouth, P. A. Wender, Proc. Natl. Acad. Sci. USA 2012, 109, 13171–13176;
- 10eE.-K. Bang, G. Gasparini, G. Molinard, A. Roux, N. Sakai, S. Matile, J. Am. Chem. Soc. 2013, 135, 2088–2091;
- 10fG. Gasparini, E.-K. Bang, G. Molinard, D. V. Tulumello, S. Ward, S. O. Kelley, A. Roux, N. Sakai, S. Matile, J. Am. Chem. Soc. 2014, 136, 6069–6074;
- 10gG. Gasparini, S. Matile, Chem. Commun. 2015, 51, 17160–17162;
- 10hC. J. McKinlay, R. M. Waymouth, P. A. Wender, J. Am. Chem. Soc. 2016, 138, 3510–3517;
- 10iE. Derivery, E. Bartolami, S. Matile, M. Gonzalez-Gaitan, J. Am. Chem. Soc. 2017, 139, 10172–10175.
- 11R. Mogaki, P. K. Hashim, K. Okuro, T. Aida, Chem. Soc. Rev. 2017, 46, 6480–6491.
- 12
- 12aK. Okuro, K. Kinbara, K. Tsumoto, N. Ishii, T. Aida, J. Am. Chem. Soc. 2009, 131, 1626–1627;
- 12bR. Mogaki, K. Okuro, R. Ueki, S. Sando, T. Aida, J. Am. Chem. Soc. 2019, 141, 8035–8040;
- 12cN. B. Hentzen, R. Mogaki, S. Otake, K. Okuro, T. Aida, J. Am. Chem. Soc. 2020, 142, 8080–8084.
- 13
- 13aT. Ozawa, K. Sasaki, Y. Umezawa, Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1999, 1434, 211–220;
- 13bL. Settimoto, S. Donnini, A. H. Juffer, R. W. Woody, O. Marin, Pept. Sci. 2007, 88, 373–385;
- 13cY. Zhou, W. Yang, M. M. Lurtz, Y. Ye, Y. Huang, H.-W. Lee, Y. Chen, C. F. Louis, J. J. Yang, J. Biol. Chem. 2007, 282, 35005–35017;
- 13dS. R. Martin, L. Masino, P. M. Bayley, Protein Sci. 2000, 9, 2477–2488;
- 13eD. Chin, A. R. Means, Trends Cell Biol. 2000, 10, 322–328.
- 14
- 14aB. O. Oyajobi, Arthritis Res. Ther. 2007, 9(Supp 1), S4;
- 14bA. Balakumaran, P. G. Robey, N. Fedarko, O. Landgren, Expert Rev. Mol. Diagn. 2010, 10, 465–480.
- 15
- 15aW. Ji, L. Xiao, Y. Ling, C. Ching, M. Matsumoto, R. P. Bisbey, D. E. Helbling, W. R. Dichtel, J. Am. Chem. Soc. 2018, 140, 12677–12681.
- 16
- 16aJ. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 2007, 36, 1249–1262;
- 16bB. K. Hughes, W. A. Braunecker, D. C. Bobela, S. U. Nanayakkara, O. G. Reid, J. C. Johnson, J. Phys. Chem. Lett. 2016, 7, 3660–3665;
- 16cS. Royuela, E. García-Garrido, M. M. Arroyo, M. J. Mancheño, M. M. Ramos, D. González-Rodríguez, Á. Somoza, F. Zamora, J. L. Segura, Chem. Commun. 2018, 54, 8729–8732.
- 17P. Fortgang, T. Tite, V. Barnier, N. Zehani, C. Maddi, F. Lagarde, A.-S. Loir, N. Jaffrezic-Renault, C. Donnet, F. Garrelie, C. Chaix, ACS Appl. Mater. Interfaces 2016, 8, 1424–1433.
- 18J. S. Stevens, A. C. de Luca, M. Pelendritis, G. Terenghi, S. Downes, S. L. M. Schroeder, Surf. Interface Anal. 2013, 45, 1238–1246.
- 19X. Li, C. Zhang, S. Cai, X. Lei, V. Altoe, F. Hong, J. J. Urban, J. Ciston, E. M. Chan, Y. Liu, Nat. Commun. 2018, 9, 2998.
- 20J. P. Collman, N. K. Devaraj, T. P. A. Eberspacher, C. E. D. Chidsey, Langmuir 2006, 22, 2457–2464.
- 21D. L. Pavia, G. M. Lampman, G. S. Kriz, J. A. Vyvyan, Introduction to Spectroscopy, Cengage Learning, Belmont, 2015, pp. 52–84.
- 22See the Supporting Information.
- 23B. Ruan, H. Liu, L. Xie, J. Fluoresc. 2020, 30, 427–435.
- 24
- 24aH. Pietsch, J. Tuchnitz, Z. Phys. Chem. 1961, 216, 372–374;
- 24bN. A. Evans, J. Soc. Dyers Colour. 1970, 86, 174–177.