Manganese-Catalyzed Asymmetric Hydrogenation of Quinolines Enabled by π–π Interaction**
Chenguang Liu
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorMingyang Wang
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorShihan Liu
Chongqing Key Laboratory of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorYujie Wang
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYong Peng
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yu Lan
Institute of Green Catalysis, College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Chongqing Key Laboratory of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qiang Liu
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorChenguang Liu
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorMingyang Wang
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorShihan Liu
Chongqing Key Laboratory of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorYujie Wang
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYong Peng
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yu Lan
Institute of Green Catalysis, College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Chongqing Key Laboratory of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qiang Liu
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv.13034363.v2).
Graphical Abstract
Abstract
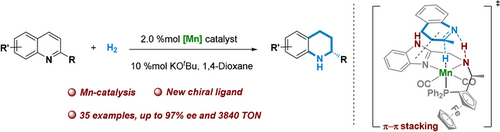
The non-noble metal-catalyzed asymmetric hydrogenation of N-heteroaromatics, quinolines, is reported. A new chiral pincer manganese catalyst showed outstanding catalytic activity in the asymmetric hydrogenation of quinolines, affording high yields and enantioselectivities (up to 97 % ee). A turnover number of 3840 was reached at a low catalyst loading (S/C=4000), which is competitive with the activity of most effective noble metal catalysts for this reaction. The precise regulation of the enantioselectivity were ensured by a π–π interaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202013540-sup-0001-misc_information.pdf14.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. Sridharan, P. A. Suryavanshi, J. C. Menéndez, Chem. Rev. 2011, 111, 7157–7259;
- 1bI. Muthukrishnan, V. Sridharan, J. C. Menéndez, Chem. Rev. 2019, 119, 5057–5191.
- 2
- 2aA. R. Katritzky, S. Rachwal, B. Rachwal, Tetrahedron 1996, 52, 15031–15070;
- 2bV. Kouznetsov, A. Palma, C. Ewert, A. Varlamov, J. Heterocycl. Chem. 1998, 35, 761–785;
- 2cM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. Int. Ed. 2006, 45, 3683–3686; Angew. Chem. 2006, 118, 3765–3768;
- 2dQ.-S. Guo, D.-M. Du, J. Xu, Angew. Chem. Int. Ed. 2008, 47, 759–762; Angew. Chem. 2008, 120, 771–774;
- 2eC. Wang, C. Li, X. Wu, A. Pettman, J. Xiao, Angew. Chem. Int. Ed. 2009, 48, 6524–6528; Angew. Chem. 2009, 121, 6646–6650;
- 2fG. D. Muñoz, G. B. Dudley, Org. Prep. Proced. Int. 2015, 47, 179–206.
- 3
- 3aD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557–2590;
- 3bY.-M. He, F.-T. Song, Q.-H. Fan, in Stereoselective Formation of Amines (Eds.: W. Li, X. Zhang), Springer, Berlin, Heidelberg, 2014, pp. 145–190;
- 3cZ. X. Giustra, J. S. A. Ishibashi, S.-Y. Liu, Coord. Chem. Rev. 2016, 314, 134–181;
- 3dY.-E. Luo, Y.-M. He, Q.-H. Fan, Chem. Rec. 2016, 16, 2697–2711;
- 3eK. Mashima, K. Higashida, A. Iimuro, H. Nagae, Y. Kita, Chem. Rec. 2016, 16, 2585–2598;
- 3fM. P. Wiesenfeldt, Z. Nairoukh, T. Dalton, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 10460–10476; Angew. Chem. 2019, 131, 10570–10586.
- 4
- 4aW.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han, Y.-G. Zhou, J. Am. Chem. Soc. 2003, 125, 10536–10537;
- 4bL. Xu, K. H. Lam, J. Ji, J. Wu, Q.-H. Fan, W.-H. Lo, A. S. C. Chan, Chem. Commun. 2005, 1390–1392;
- 4cS.-M. Lu, Y.-Q. Wang, X.-W. Han, Y.-G. Zhou, Angew. Chem. Int. Ed. 2006, 45, 2260–2263; Angew. Chem. 2006, 118, 2318–2321;
- 4dL. Qiu, F. Y. Kwong, J. Wu, W. H. Lam, S. Chan, W.-Y. Yu, Y.-M. Li, R. Guo, Z. Zhou, A. S. C. Chan, J. Am. Chem. Soc. 2006, 128, 5955–5965;
- 4eM. T. Reetz, X. Li, Chem. Commun. 2006, 2159–2160;
- 4fW.-J. Tang, S.-F. Zhu, L.-J. Xu, Q.-L. Zhou, Q.-H. Fan, H.-F. Zhou, K. Lam, A. S. C. Chan, Chem. Commun. 2007, 613–615;
- 4gX.-B. Wang, Y.-G. Zhou, J. Org. Chem. 2008, 73, 5640–5642;
- 4hH. Tadaoka, D. Cartigny, T. Nagano, T. Gosavi, T. Ayad, J.-P. Genêt, T. Ohshima, V. Ratovelomanana-Vidal, K. Mashima, Chem. Eur. J. 2009, 15, 9990–9994;
- 4iW.-J. Tang, J. Tan, L.-J. Xu, K.-H. Lam, Q.-H. Fan, A. S. C. Chan, Adv. Synth. Catal. 2010, 352, 1055–1062.
- 5
- 5aH. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q.-H. Fan, J. Pan, L. Gu, A. S. C. Chan, Angew. Chem. Int. Ed. 2008, 47, 8464–8467; Angew. Chem. 2008, 120, 8592–8595;
- 5bZ.-J. Wang, H.-F. Zhou, T.-L. Wang, Y.-M. He, Q.-H. Fan, Green Chem. 2009, 11, 767–769;
- 5cT. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu, A. S. C. Chan, J. Am. Chem. Soc. 2011, 133, 9878–9891;
- 5dW. Ma, J. Zhang, C. Xu, F. Chen, Y.-M. He, Q.-H. Fan, Angew. Chem. Int. Ed. 2016, 55, 12891–12894; Angew. Chem. 2016, 128, 13083–13086;
- 5eY. Chen, Y.-M. He, S. Zhang, T. Miao, Q.-H. Fan, Angew. Chem. Int. Ed. 2019, 58, 3809–3813; Angew. Chem. 2019, 131, 3849–3853;
- 5fY. Chen, Y. Pan, Y.-M. He, Q.-H. Fan, Angew. Chem. Int. Ed. 2019, 58, 16831–16834; Angew. Chem. 2019, 131, 16987–16990.
- 6J. Wen, R. Tan, S. Liu, Q. Zhao, X. Zhang, Chem. Sci. 2016, 7, 3047–3051.
- 7X.-F. Cai, W.-X. Huang, Z.-P. Chen, Y.-G. Zhou, Chem. Commun. 2014, 50, 9588–9590.
- 8
- 8aZ. Zhang, H. Du, Org. Lett. 2015, 17, 2816–2819;
- 8bZ. Zhang, H. Du, Org. Lett. 2015, 17, 6266–6269;
- 8cZ. Zhang, H. Du, Angew. Chem. Int. Ed. 2015, 54, 623–626; Angew. Chem. 2015, 127, 633–636;
- 8dX. Li, J.-J. Tian, N. Liu, X.-S. Tu, N.-N. Zeng, X.-C. Wang, Angew. Chem. Int. Ed. 2019, 58, 4664–4668; Angew. Chem. 2019, 131, 4712–4716.
- 9
- 9aR. H. Morris, Chem. Soc. Rev. 2009, 38, 2282–2291;
- 9bW. Zuo, A. J. Lough, Y. F. Li, R. H. Morris, Science 2013, 342, 1080–1083;
- 9cP. O. Lagaditis, P. E. Sues, J. F. Sonnenberg, K. Y. Wan, A. J. Lough, R. H. Morris, J. Am. Chem. Soc. 2014, 136, 1367–1380;
- 9dP. J. Chirik, Acc. Chem. Res. 2015, 48, 1687–1695;
- 9eR. H. Morris, Acc. Chem. Res. 2015, 48, 1494–1502;
- 9fR. Arevalo, P. J. Chirik, J. Am. Chem. Soc. 2019, 141, 9106–9123;
- 9gC. S. G. Seo, R. H. Morris, Organometallics 2019, 38, 47–65.
- 10
- 10aS. Monfette, Z. R. Turner, S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2012, 134, 4561–4564;
- 10bM. R. Friedfeld, M. Shevlin, J. M. Hoyt, S. W. Krska, M. T. Tudge, P. J. Chirik, Science 2013, 342, 1076–1080;
- 10cJ. Chen, C. Chen, C. Ji, Z. Lu, Org. Lett. 2016, 18, 1594–1597;
- 10dJ. Guo, B. Cheng, X. Shen, Z. Lu, J. Am. Chem. Soc. 2017, 139, 15316–15319;
- 10eJ. Guo, X. Shen, Z. Lu, Angew. Chem. Int. Ed. 2017, 56, 615–618; Angew. Chem. 2017, 129, 630–633;
- 10fM. R. Friedfeld, H. Zhong, R. T. Ruck, M. Shevlin, P. J. Chirik, Science 2018, 360, 888–893;
- 10gA. Mukherjee, D. Milstein, ACS Catal. 2018, 8, 11435–11469;
- 10hW. Ai, R. Zhong, X. Liu, Q. Liu, Chem. Rev. 2019, 119, 2876–2953;
- 10iY. Hu, Z. Zhang, J. Zhang, Y. Liu, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2019, 58, 15767–15771; Angew. Chem. 2019, 131, 15914–15918;
- 10jX. Du, Y. Xiao, J.-M. Huang, Y. Zhang, Y.-N. Duan, H. Wang, C. Shi, G.-Q. Chen, X. Zhang, Nat. Commun. 2020, 11, 3239;
- 10kH. Zhong, M. Shevlin, P. J. Chirik, J. Am. Chem. Soc. 2020, 142, 5272–5281.
- 11
- 11aM. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562–3569;
- 11bW. Gao, H. Lv, T. Zhang, Y. Yang, L. W. Chung, Y.-D. Wu, X. Zhang, Chem. Sci. 2017, 8, 6419–6422;
- 11cY.-Q. Guan, Z. Han, X. Li, C. You, X. Tan, H. Lv, X. Zhang, Chem. Sci. 2019, 10, 252–256;
- 11dY. Hu, J. Chen, B. Li, Z. Zhang, I. D. Gridnev, W. Zhang, Angew. Chem. Int. Ed. 2020, 59, 5371–5375; Angew. Chem. 2020, 132, 5409–5413.
- 12Z. Zhang, N. A. Butt, M. Zhou, D. Liu, W. Zhang, Chin. J. Chem. 2018, 36, 443–454.
- 13
- 13aD. A. Valyaev, G. Lavigne, N. Lugan, Coord. Chem. Rev. 2016, 308, 191–235;
- 13bG. A. Filonenko, R. van Putten, E. J. M. Hensen, E. A. Pidko, Chem. Soc. Rev. 2018, 47, 1459–1483;
- 13cF. Kallmeier, R. Kempe, Angew. Chem. Int. Ed. 2018, 57, 46–60; Angew. Chem. 2018, 130, 48–63;
- 13dL. Alig, M. Fritz, S. Schneider, Chem. Rev. 2019, 119, 2681–2751.
- 14
- 14aS. Elangovan, M. Garbe, H. Jiao, A. Spannenberg, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2016, 55, 15364–15368; Angew. Chem. 2016, 128, 15590–15594;
- 14bV. Papa, J. R. Cabrero-Antonino, E. Alberico, A. Spanneberg, K. Junge, H. Junge, M. Beller, Chem. Sci. 2017, 8, 3576–3585;
- 14cM. Glatz, B. Stöger, D. Himmelbauer, L. F. Veiros, K. Kirchner, ACS Catal. 2018, 8, 4009–4016;
- 14dA. Kaithal, M. Hölscher, W. Leitner, Angew. Chem. Int. Ed. 2018, 57, 13449–13453; Angew. Chem. 2018, 130, 13637–13641;
- 14eA. Kumar, T. Janes, N. A. Espinosa-Jalapa, D. Milstein, Angew. Chem. Int. Ed. 2018, 57, 12076–12080; Angew. Chem. 2018, 130, 12252–12256;
- 14fV. Zubar, Y. Lebedev, L. M. Azofra, L. Cavallo, O. El-Sepelgy, M. Rueping, Angew. Chem. Int. Ed. 2018, 57, 13439–13443; Angew. Chem. 2018, 130, 13627–13631;
- 14gU. K. Das, A. Kumar, Y. Ben-David, M. A. Iron, D. Milstein, J. Am. Chem. Soc. 2019, 141, 12962–12966;
- 14hF. Freitag, T. Irrgang, R. Kempe, J. Am. Chem. Soc. 2019, 141, 11677–11685;
- 14iV. Papa, Y. Cao, A. Spannenberg, K. Junge, M. Beller, Nat. Catal. 2020, 3, 135–142;
- 14jZ. Wang, L. Chen, G. Mao, C. Wang, Chin. Chem. Lett. 2020, 31, 1890–1894.
- 15S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge, M. Beller, J. Am. Chem. Soc. 2016, 138, 8809–8814.
- 16
- 16aM. Garbe, K. Junge, S. Walker, Z. Wei, H. Jiao, A. Spannenberg, S. Bachmann, M. Scalone, M. Beller, Angew. Chem. Int. Ed. 2017, 56, 11237–11241; Angew. Chem. 2017, 129, 11389–11393;
- 16bM. B. Widegren, G. J. Harkness, A. M. Z. Slawin, D. B. Cordes, M. L. Clarke, Angew. Chem. Int. Ed. 2017, 56, 5825–5828; Angew. Chem. 2017, 129, 5919–5922;
- 16cL. Zhang, Y. Tang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2019, 58, 4973–4977; Angew. Chem. 2019, 131, 5027–5031;
- 16dL. Zhang, Z. Wang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2020, 59, 15565–15569; Angew. Chem. 2020, 132, 15695–15699.
- 17
- 17aS. Fu, Z. Shao, Y. Wang, Q. Liu, J. Am. Chem. Soc. 2017, 139, 11941–11948;
- 17bY. Wang, Z. Shao, K. Zhang, Q. Liu, Angew. Chem. Int. Ed. 2018, 57, 15143–15147; Angew. Chem. 2018, 130, 15363–15367;
- 17cY. Wang, L. Zhu, Z. Shao, G. Li, Y. Lan, Q. Liu, J. Am. Chem. Soc. 2019, 141, 17337–17349;
- 17dZ. Shao, Y. Li, C. Liu, W. Ai, S.-P. Luo, Q. Liu, Nat. Commun. 2020, 11, 591.
- 18Y. He, Q. Fan, Chin. J. Org. Chem. 2019, 39, 3310–3311.
- 19F. Ling, H. Hou, J. Chen, S. Nian, X. Yi, Z. Wang, D. Song, W. Zhong, Org. Lett. 2019, 21, 3937–3941.
- 20V. I. Minkin, A. D. Garnovskii, J. Elguero, A. R. Katritzky, O. V. Denisko, in Advances in Heterocyclic Chemistry, Vol. 76 (Ed.: A. R. Katritzky), Academic Press, San Diego, 2000, pp. 157–323.
- 21
- 21aB. Zhao, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2013, 52, 4744–4788; Angew. Chem. 2013, 125, 4844–4889;
- 21bP. A. Dub, B. L. Scott, J. C. Gordon, J. Am. Chem. Soc. 2017, 139, 1245–1260.
- 22J. Bálint, G. Egri, E. Fogassy, Z. Böcskei, K. Simon, A. Gajáry, A. Friesz, Tetrahedron: Asymmetry 1999, 10, 1079–1087.
- 23I. Jacquemond-Collet, F. Benoit-Vical, M. Valentin, A. Stanislas, E. Mallié, I. Fourasté, Planta Med. 2002, 68, 68–69.
- 24B. Li, C. Xu, Y.-M. He, G.-J. Deng, Q.-H. Fan, Chin. J. Chem. 2018, 36, 1169–1173.
- 25T. Sammi, O. Silakari, M. Ravikumar, Chem. Biol. Drug Des. 2008, 71, 155–166.
- 26T. Asberom, H. S. Guzik, H. B. Josien, D. A. Pissarnitski, patent WO2003014075A2, 2003.