Emerging Cubic Chirality in γCD-MOF for Fabricating Circularly Polarized Luminescent Crystalline Materials and the Size Effect
Liangyu Hu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorKun Li
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorWeili Shang
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Dr. Xuefeng Zhu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Minghua Liu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Department Collaborative Innovation Centre of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorLiangyu Hu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorKun Li
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorWeili Shang
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Dr. Xuefeng Zhu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Minghua Liu
Beijing National Laboratory for Molecular Science, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, ZhongGuanCun North First Street 2, Beijing, 100190 China
Department Collaborative Innovation Centre of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorGraphical Abstract
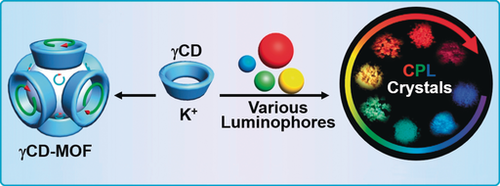
The host–guest interaction between the chiral void of γCD-MOF and achiral luminophores with different charges and sizes are presented. Numerous achiral luminophores could be integrated into γCD-MOF and emitted significantly boosted positive or negative circularly polarized luminescence (CPL). When the size of the guest luminophores was close to the cube size, strong negative CPL was observed.
Abstract
The chiral feature of γCD-MOF, and especially the emergent cubic void, was not unveiled so far. Now, through the host–guest interaction between γCD-MOF and achiral luminophores with different charges and sizes, the unique cubic chirality of the emerging void in γCD-MOF as well as a size effect on CPL induction are revealed for the first time. Numerous achiral luminophores could be integrated into γCD-MOF and emitted significantly boosted circularly polarized luminescence. While the small sized luminophores preferred to be loaded into the intrinsic void of γCD, large ones were selectively encapsulated into the cubic void. Interestingly, when the size of the guest luminophores was close to the cube size, it showed strong negative CPL. Otherwise, either positive or negative CPL was induced.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202000589-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Ruiz-Mirazo, C. Briones, A. de la Escosura, Chem. Rev. 2014, 114, 285–366;
- 1bM. Liu, L. Zhang, T. Wang, Chem. Rev. 2015, 115, 7304–7397;
- 1cE. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai, K. Maeda, Chem. Rev. 2016, 116, 13752–13990.
- 2
- 2aS. Allenmark, Chirality 2003, 15, 409–422;
- 2bG. Crini, Chem. Rev. 2014, 114, 10940–10975;
- 2cD. Prochowicz, A. Kornowicz, J. Lewinski, Chem. Rev. 2017, 117, 13461–13501.
- 3
- 3aH. Furukawa, K. E. Cordova, M. O'Keeffe, O. M. Yaghi, Science 2013, 341, 1230444;
- 3bY. Takashima, V. Martinez Martinez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto, S. Kitagawa, Nat. Commun. 2011, 2, 168;
- 3cY. Cui, Y. Yue, G. Qian, B. Chen, Chem. Rev. 2012, 112, 1126–1162;
- 3dM. D. Allendorf, C. A. Bauer, R. K. Bhakta, R. J. T. Houk, Chem. Soc. Rev. 2009, 38, 1330–1352;
- 3eZ. Hu, B. J. Deibert, J. Li, Chem. Soc. Rev. 2014, 43, 5815–5840;
- 3fR. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang, H.-C. Zhou, Coord. Chem. Rev. 2009, 253, 3042–3066.
- 4
- 4aR. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi, J. F. Stoddart, Angew. Chem. Int. Ed. 2010, 49, 8630–8634; Angew. Chem. 2010, 122, 8812–8816;
- 4bS. Han, Y. Wei, C. Valente, R. S. Forgan, J. J. Gassensmith, R. A. Smaldone, H. Nakanishi, A. Coskun, J. F. Stoddart, B. A. Grzybowski, Angew. Chem. Int. Ed. 2011, 50, 276–279; Angew. Chem. 2011, 123, 290–293.
- 5
- 5aY. Wei, S. Han, D. A. Walker, P. E. Fuller, B. A. Grzybowski, Angew. Chem. Int. Ed. 2012, 51, 7435–7439; Angew. Chem. 2012, 124, 7553–7557;
- 5bS. Han, Y. Wei, B. A. Grzybowski, Chem. Eur. J. 2013, 19, 11194–11198;
- 5cZ. Liu, J. F. Stoddart, Pure Appl. Chem. 2014, 86, 1323–1334;
- 5dT. Rajkumar, D. Kukkar, K.-H. Kim, J. R. Sohn, A. Deep, J. Ind. Eng. Chem. 2019, 72, 50–66;
- 5eY. Chen, B. Yu, Y. Cui, S. Xu, J. Gong, Chem. Mater. 2019, 31, 1289–1295;
- 5fK. J. Hartlieb, J. M. Holcroft, P. Z. Moghadam, N. A. Vermeulen, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, R. Q. Snurr, J. F. Stoddart, J. Am. Chem. Soc. 2016, 138, 2292–2301;
- 5gC.-X. Yang, Y.-Z. Zheng, X.-P. Yan, RSC Adv. 2017, 7, 36297–36301.
- 6
- 6aH. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori, T. Kawai, J. Am. Chem. Soc. 2011, 133, 9266–9269;
- 6bK. Okano, M. Taguchi, M. Fujiki, T. Yamashita, Angew. Chem. Int. Ed. 2011, 50, 12474–12477; Angew. Chem. 2011, 123, 12682–12685;
- 6cJ. Kumar, T. Nakashima, T. Kawai, J. Phys. Chem. Lett. 2015, 6, 3445–3452;
- 6dE. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz, S. de la Moya, Chem. Eur. J. 2015, 21, 13488–13500;
- 6eG. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo, S. Abbate, Chirality 2016, 28, 696–707;
- 6fN. Hellou, M. Srebro-Hooper, L. Favereau, F. Zinna, E. Caytan, L. Toupet, V. Dorcet, M. Jean, N. Vanthuyne, J. A. G. Williams, L. Di Bari, J. Autschbach, J. Crassous, Angew. Chem. Int. Ed. 2017, 56, 8236–8239; Angew. Chem. 2017, 129, 8348–8351;
- 6gL. Wang, L. Yin, W. Zhang, X. Zhu, M. Fujiki, J. Am. Chem. Soc. 2017, 139, 13218–13226;
- 6hJ. F. Koegel, S. Kusaka, R. Sakamoto, T. Iwashima, M. Tsuchiya, R. Toyoda, R. Matsuoka, T. Tsukamoto, J. Yuasa, Y. Kitagawa, T. Kawai, H. Nishihara, Angew. Chem. Int. Ed. 2016, 55, 1377–1381; Angew. Chem. 2016, 128, 1399–1403;
- 6iZ. Shen, T. Wang, L. Shi, Z. Tang, M. Liu, Chem. Sci. 2015, 6, 4267–4272.
- 7
- 7aR. Carr, N. H. Evans, D. Parker, Chem. Soc. Rev. 2012, 41, 7673–7686;
- 7bJ. Han, S. Guo, H. Lu, S. Liu, Q. Zhao, W. Huang, Adv. Opt. Mater. 2018, 6, 1800538;
- 7cY. Sang, J. Han, T. Zhao, P. Duan, M. Liu, Adv. Mater. 2019, https://doi.org/10.1002/adma.201900110.
- 8
- 8aK. Takaishi, K. Iwachido, R. Takehana, M. Uchiyama, T. Ema, J. Am. Chem. Soc. 2019, 141, 6185–6190;
- 8bT. Zhao, J. Han, X. Jin, Y. Liu, M. Liu, P. Duan, Angew. Chem. Int. Ed. 2019, 58, 4978–4982; Angew. Chem. 2019, 131, 5032–5036;
- 8cE. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, M. J. Ortiz, B. G. Vo, G. Muller, S. de la Moya, J. Am. Chem. Soc. 2014, 136, 3346–3349;
- 8dC.-T. Yeung, K.-H. Yim, H.-Y. Wong, R. Pal, W.-S. Lo, S.-C. Yan, M. Y.-M. Wong, D. Yufit, D. E. Smiles, L. J. McCormick, S. J. Teat, D. K. Shuh, W.-T. Wong, G.-L. Law, Nat. Commun. 2017, 8, 1128.
- 9
- 9aT. Goto, Y. Okazaki, M. Ueki, Y. Kuwahara, M. Takafuji, R. Oda, H. Ihara, Angew. Chem. Int. Ed. 2017, 56, 2989–2993; Angew. Chem. 2017, 129, 3035–3039;
- 9bY. Okazaki, N. Ryu, T. Buffeteau, S. Pathan, S. Nagaoka, E. Pouget, S. Nlate, H. Ihara, R. Oda, Chem. Commun. 2018, 54, 10244–10247;
- 9cJ. Zhang, W. Feng, H. Zhang, Z. Wang, H. A. Calcaterra, B. Yeom, P. A. Hu, N. A. Kotov, Nat. Commun. 2016, 7, 10701;
- 9dX. Li, W. Hu, Y. Wang, Y. Quan, Y. Cheng, Chem. Commun. 2019, 55, 5179–5182;
- 9eJ. Han, J. You, X. Li, P. Duan, M. Liu, Adv. Mater. 2017, 29, 1606503;
- 9fQ. Jiang, X. Xu, P.-A. Yin, K. Ma, Y. Zhen, P. Duan, Q. Peng, W.-Q. Chen, B. Ding, J. Am. Chem. Soc. 2019, 141, 9490–9494;
- 9gC. Li, J. Cho, K. Yamada, D. Hashizume, F. Araoka, H. Takezoe, T. Aida, Y. Ishida, Nat. Commun. 2015, 6, 8418;
- 9hY. Mimura, S. Kitamura, M. Shizuma, M. Kitamatsu, M. Fujiki, Y. Imai, ChemistrySelect 2017, 2, 7759–7764;
- 9iY. Jin, S. Li, Z. Han, B.-J. Yan, H.-Y. Li, X.-Y. Dong, S.-Q. Zang, Angew. Chem. Int. Ed. 2019, 58, 12143–12148; Angew. Chem. 2019, 131, 12271–12276.
- 10M. Zhou, G. P. Robertson, J. Roovers, Inorg. Chem. 2005, 44, 8317–8325.
- 11
- 11aR. Rubires, J. Crusats, Z. El-Hachemi, T. Jaramillo, M. Lopez, E. Valls, J. A. Farrera, J. M. Ribo, New J. Chem. 1999, 23, 189–198;
- 11bK. Sasaki, H. Nakagawa, X. Y. Zhang, S. Sakurai, K. Kano, Y. Kuroda, Chem. Commun. 2004, 408–409.
- 12R. Watanabe, N. Hara, Y. Imai, M. Hasegawa, S. Ishioka, Y. Mazaki, K.-i. Sugiura, ChemistrySelect 2018, 3, 3576–3581.
- 13
- 13aF. Li, Y. Li, G. Wei, Y. Wang, S. Li, Y. Cheng, Chem. Eur. J. 2016, 22, 12910–12915;
- 13bJ. Kumar, H. Tsumatori, J. Yuasa, T. Kawai, T. Nakashima, Angew. Chem. Int. Ed. 2015, 54, 5943–5947; Angew. Chem. 2015, 127, 6041–6045.
- 14J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Chem. Rev. 2015, 115, 11718–11940.
- 15
- 15aJ. Roose, B. Z. Tang, K. S. Wong, Small 2016, 12, 6495–6512;
- 15bX. Liu, J. Jiao, X. Jiang, J. Li, Y. Cheng, C. Zhu, J. Mater. Chem. C 2013, 1, 4713–4719;
- 15cS. Zhang, Y. Sheng, G. Wei, Y. Quan, Y. Cheng, C. Zhu, Polym. Chem. 2015, 6, 2416–2422.
- 16
- 16aA. Thomas, T. Chervy, S. Azzini, M. H. Li, J. George, C. Genet, T. W. Ebbesen, J. Phys. Chem. C 2018, 122, 14205–14212;
- 16bH. Gillgren, A. Stenstam, M. Ardhammar, B. Nordón, E. Sparr, S. Ulvenlund, Langmuir 2002, 18, 462–469;
- 16cA. Ohira, K. Okoshi, M. Fujiki, M. Kunitake, M. Naito, Adv. Mater. 2004, 16, 1645–1650;
- 16dG. Gottarelli, S. Lena, S. Masiero, S. Pieraccini, G. P. Spada, Chirality 2008, 20, 471–485.
- 17
- 17aH. G. Brittain, Chem. Phys. Lett. 1981, 83, 161–164;
- 17bK. Kano, H. Matsumoto, S. Hashimoto, M. Sisido, Y. Imanishi, J. Am. Chem. Soc. 1985, 107, 6117–6118;
- 17cM. Inouye, K. Hayashi, Y. Yonenaga, T. Itou, K. Fujimoto, T. Uchida, M. Iwamura, K. Nozaki, Angew. Chem. Int. Ed. 2014, 53, 14392–14396; Angew. Chem. 2014, 126, 14620–14624;
- 17dK. Hayashi, Y. Miyaoka, Y. Ohishi, T.-A. Uchida, M. Iwamura, K. Nozaki, M. Inouye, Chem. Eur. J. 2018, 24, 14613–14616.