Hydrotrifluoromethylthiolation of Unactivated Alkenes and Alkynes with Trifluoromethanesulfonic Anhydride through Deoxygenative Reduction and Photoredox Radical Processes
Yao Ouyang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorDr. Xiu-Hua Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng-Ling Qing
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Key Laboratory of Science and Technology of Eco-Textiles, Ministry of Education, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Lu, Shanghai, 201620 China
Search for more papers by this authorYao Ouyang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorDr. Xiu-Hua Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng-Ling Qing
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Science, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Key Laboratory of Science and Technology of Eco-Textiles, Ministry of Education, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Lu, Shanghai, 201620 China
Search for more papers by this authorGraphical Abstract
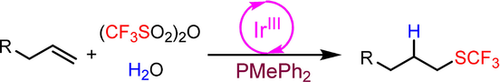
A practical anti-Markovnikov hydrotrifluoromethylthiolation of unactivated alkenes using (CF3SO2)2O and H2O as the SCF3 and H sources was achieved through deoxygenative reduction with PMePh2 and photoredox radical processes. This reaction is the first example of using (CF3SO2)2O as a trifluoromethylthiolating reagent and provides a new strategy for radical trifluoromethylthiolation.
Abstract
An ongoing challenge in trifluoromethylthiolation reactions is the use of less expensive and easily available trifluoromethylthio sources. Herein, we disclose an unprecedented usage of trifluoromethanesulfonic anhydride (Tf2O) as a radical trifluoromethylthiolating reagent. Hydrotrifluoromethylthiolation of unactivated alkenes and alkynes with Tf2O in the presence of PMePh2 and H2O under visible-light photoredox catalysis gave the addition products. The trifluoromethylthio radical (.SCF3) was first formed from Tf2O through a photoredox radical processes and deoxygenative reduction of PMePh2, and H2O serves as the H-atom donor for the hydrotrifluoromethylthiolation reaction. This reaction provides a new strategy for radical trifluoromethylthiolation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201911323-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320;
- 1bJ. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432;
- 1cN. A. Meanwell, J. Med. Chem. 2018, 61, 5822.
- 2Selected reviews:
- 2aT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214; Angew. Chem. 2013, 125, 8372;
- 2bL. Chu, F.-L. Qing, Acc. Chem. Res. 2014, 47, 1513;
- 2cF. Toulgoat, S. Alazet, T. Billard, Eur. J. Org. Chem. 2014, 2415;
- 2dX. Shao, C. Xu, L. Lu, Q. Shen, Acc. Chem. Res. 2015, 48, 1227;
- 2eX.-H. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115, 731;
- 2fX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826;
- 2gS. Barata-Vallejo, S. Bonesi, A. Postigo, Org. Biomol. Chem. 2016, 14, 7150;
- 2hH. Zheng, Y. Huang, Z. Weng, Tetrahedron Lett. 2016, 57, 1397.
- 3
- 3aH. Chachignon, D. Cahard, Chin. J. Chem. 2016, 34, 445;
- 3bJ. Zhang, J.-D. Yang, H. Zheng, X.-S. Xue, H. Mayr, J.-P. Cheng, Angew. Chem. Int. Ed. 2018, 57, 12690; Angew. Chem. 2018, 130, 12872.
- 4
- 4aA. Ferry, T. Billard, B. R. Langlois, E. Bacqué, Angew. Chem. Int. Ed. 2009, 48, 8551; Angew. Chem. 2009, 121, 8703;
- 4bT. Bootwicha, X. Liu, R. Pluta, I. Atodiresei, M. Rueping, Angew. Chem. Int. Ed. 2013, 52, 12856; Angew. Chem. 2013, 125, 13093;
- 4cC. Xu, B. Ma, Q. Shen, Angew. Chem. Int. Ed. 2014, 53, 9316; Angew. Chem. 2014, 126, 9470;
- 4dS. Alazet, L. Zimmer, T. Billard, Chem. Eur. J. 2014, 20, 8589;
- 4eP. Zhang, M. Li, X. S. Xue, C. Xu, Q. Zhao, Y. Liu, H. Y. Wang, Y. Guo, L. Lu, Q. Shen, J. Org. Chem. 2016, 81, 7486.
- 5
- 5aX. Shao, X. Wang, T. Yang, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 2013, 52, 3457; Angew. Chem. 2013, 125, 3541;
- 5bE. V. Vinogradova, P. Müller, S. L. Buchwald, Angew. Chem. Int. Ed. 2014, 53, 3125; Angew. Chem. 2014, 126, 3189;
- 5cX. Shao, C. Xu, L. Lu, Q. Shen, J. Org. Chem. 2015, 80, 3012.
- 6
- 6aY. D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro, N. Shibata, J. Am. Chem. Soc. 2013, 135, 8782;
- 6bZ. Huang, Y. D. Yang, E. Tokunaga, N. Shibata, Org. Lett. 2015, 17, 1094;
- 6cS. Arimori, M. Takada, N. Shibata, Org. Lett. 2015, 17, 1063.
- 7
- 7aL. Jiang, J. Qian, W. Yi, G. Lu, C. Cai, W. Zhang, Angew. Chem. Int. Ed. 2015, 54, 14965; Angew. Chem. 2015, 127, 15178;
- 7bY. Yang, L. Xu, S. Yu, X. Liu, Y. Zhang, D. A. Vicic, Chem. Eur. J. 2016, 22, 858;
- 7cJ. Liu, X. Zhao, L. Jiang, W. Yi, Adv. Synth. Catal. 2018, 360, 4012.
- 8
- 8aH. Chachignon, M. Maeno, H. Kondo, N. Shibata, D. Cahard, Org. Lett. 2016, 18, 2467;
- 8bL. Jiang, T. Ding, W. Yi, X. Zeng, W. Zhang, Org. Lett. 2018, 20, 2236;
- 8cA. Ghosh, M. Lecomte, S.-H. Kim-Lee, A. T. Radosevich, Angew. Chem. Int. Ed. 2019, 58, 2864; Angew. Chem. 2019, 131, 2890.
- 9
- 9aJ.-Y. Guo, R.-H. Dai, W.-C. Xu, R.-X. Wu, S.-K. Tian, Chem. Commun. 2018, 54, 8980;
- 9bK. Lu, Q. Li, X. Xi, Y. Huang, Z. Gong, P. Yu, X. Zhao, Org. Chem. Front. 2018, 5, 3088.
- 10
- 10aJ. B. Hendrickson, D. D. Sternbach, K. W. Bair, Acc. Chem. Res. 1977, 10, 306;
- 10bB. A. Shainyan, L. L. Tolstikova, Chem. Rev. 2013, 113, 699.
- 11Y. Ouyang, X.-H. Xu, F.-L. Qing, Angew. Chem. Int. Ed. 2018, 57, 6926; Angew. Chem. 2018, 130, 7042.
- 12
- 12aQ.-Y. Lin, X.-H. Xu, K. Zhang, F.-L. Qing, Angew. Chem. Int. Ed. 2016, 55, 1479; Angew. Chem. 2016, 128, 1501;
- 12bQ.-Y. Lin, Y. Ran, X.-H. Xu, F.-L. Qing, Org. Lett. 2016, 18, 2419;
- 12cN. B. Heine, A. Studer, Org. Lett. 2017, 19, 4150.
- 13Selected examples:
- 13aX. Wu, L. Chu, F.-L. Qing, Angew. Chem. Int. Ed. 2013, 52, 2198; Angew. Chem. 2013, 125, 2254;
- 13bS. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Médebielle, V. Gouverneur, J. Am. Chem. Soc. 2013, 135, 2505;
- 13cD. J. Wilger, N. J. Gesmundo, D. A. Nicewicz, Chem. Sci. 2013, 4, 3160;
- 13dP. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan, X.-Y. Liu, Angew. Chem. Int. Ed. 2014, 53, 11890; Angew. Chem. 2014, 126, 12084;
- 13eS. Choi, Y. J. Kim, S. M. Kim, J. W. Yang, S. W. Kim, E. J. Cho, Nat. Commun. 2014, 5, 4881;
- 13fN. J. W. Straathof, S. E. Cramer, V. Hessel, T. Noël, Angew. Chem. Int. Ed. 2016, 55, 15549; Angew. Chem. 2016, 128, 15778;
- 13gG. H. Lonca, D. Y. Ong, T. M. H. Tran, C. Tejo, S. Chiba, F. Gagosz, Angew. Chem. Int. Ed. 2017, 56, 11440; Angew. Chem. 2017, 129, 11598;
- 13hW. Zhang, Z. Zou, Y. Wang, Y. Wang, Y. Liang, Z. Wu, Y. Zheng, Y. Pan, Angew. Chem. Int. Ed. 2019, 58, 624; Angew. Chem. 2019, 131, 634;
- 13iJ.-X. Xiang, Y. Ouyang, X.-H. Xu, F.-L. Qing, Angew. Chem. Int. Ed. 2019, 58, 10320; Angew. Chem. 2019, 131, 10426.
- 14Selected examples:
- 14aG. Ma, W. Wan, J. Li, Q. Hu, H. Jiang, S. Zhu, J. Wang, J. Hao, Chem. Commun. 2014, 50, 9749;
- 14bC. Yu, N. Iqbal, S. Park, E. J. Cho, Chem. Commun. 2014, 50, 12884;
- 14cQ.-Y. Lin, X.-H. Xu, F.-L. Qing, Org. Biomol. Chem. 2015, 13, 8740;
- 14dW. Yu, X.-H. Xu, F.-L. Qing, Org. Lett. 2016, 18, 5130;
- 14eB. Yang, X.-H. Xu, F.-L. Qing, Org. Lett. 2016, 18, 5956;
- 14fW. Huang, W. Chen, G. Wang, J. Li, X. Cheng, G. Li, ACS Catal. 2016, 6, 7471;
- 14gX. Nie, C. Cheng, G. Zhu, Angew. Chem. Int. Ed. 2017, 56, 1898; Angew. Chem. 2017, 129, 1924;
- 14hW. Shu, E. Merino, C. Nevado, ACS Catal. 2018, 8, 6401;
- 14iH. Wang, N. T. Jui, J. Am. Chem. Soc. 2018, 140, 163.
- 15A review:
- 15aA.-L. Barthelemy, E. Magnier, G. Dagousset, Synthesis 2018, 50, 4765; for selected examples, see:
- 15bK. Zhang, J.-B. Liu, F.-L. Qing, Chem. Commun. 2014, 50, 14157;
- 15cF. Yin, X.-S. Wang, Org. Lett. 2014, 16, 1128;
- 15dN. Fuentes, W. Kong, L. Fernández-Sánchez, E. Merino, C. Nevado, J. Am. Chem. Soc. 2015, 137, 964;
- 15eD.-P. Jin, P. Gao, D.-Q. Chen, S. Chen, J. Wang, X.-Y. Liu, Y.-M. Liang, Org. Lett. 2016, 18, 3486;
- 15fM. Ji, Z. Wu, J. Yu, X. Wan, C. Zhu, Adv. Synth. Catal. 2017, 359, 1959;
- 15gC.-H. Guo, D.-Q. Chen, S. Chen, X.-Y. Liu, Adv. Synth. Catal. 2017, 359, 2901;
- 15hH. Li, S. Liu, Y. Huang, X.-H. Xu, F.-L. Qing, Chem. Commun. 2017, 53, 10136;
- 15iS. Pan, H. Li, Y. Huang, X.-H. Xu, F.-L. Qing, Org. Lett. 2017, 19, 3247;
- 15jR. Honeker, R. A. Garza-Sanchez, M. N. Hopkinson, F. Glorius, Chem. Eur. J. 2016, 22, 4395;
- 15kY. Li, T. Koike, M. Akita, Asian J. Org. Chem. 2017, 6, 445;
- 15lG. Dagousset, C. Simon, E. Anselmi, B. Tuccio, T. Billard, E. Magnier, Chem. Eur. J. 2017, 23, 4282; for a theoretical study on the generation of the SCF3 radical from electrophilic reagents, see:
- 15mM. Li, B. Zhou, X.-S. Xue, J.-P. Cheng, J. Org. Chem. 2017, 82, 8697.
- 16J. F. Harris, Jr., F. W. Stacey, J. Am. Chem. Soc. 1961, 83, 840.
- 17
- 17aT. Yang, L. Lu, Q. Shen, Chem. Commun. 2015, 51, 5479;
- 17bX. Shao, X. Hong, L. Lu, Q. Shen, Tetrahedron 2019, 75, 4156.
- 18W. Wu, W. Dai, X. Ji, S. Cao, Org. Lett. 2016, 18, 2918.
- 19
- 19aA. Lopusiński, Phosphorus Sulfur Silicon Relat. Elem. 1989, 45, 137;
- 19bN. Santschi, A. Togni, J. Org. Chem. 2011, 76, 4189.
- 20
- 20aJ. B. Hendrickson, S. M. Schwartzman, Tetrahedron Lett. 1975, 16, 177;
- 20bJ. B. Hendrickson, M. S. Hussoin, J. Org. Chem. 1989, 54, 1144.
- 21A. Singh, K. Teegardin, M. Kelly, K. S. Prasad, S. Krishnan, J. D. Weaver, J. Organomet. Chem. 2015, 776, 51.
- 22G. Pandey, D. Pooranchand, U. T. Bhalerao, Tetrahedron 1991, 47, 1745.
- 23
- 23aC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322;
- 23bL.-L. Liao, G.-M. Cao, J.-H. Ye, G.-Q. Sun, W.-J. Zhou, Y.-Y. Gui, S.-S. Yan, G. Shen, D.-G. Yu, J. Am. Chem. Soc. 2018, 140, 17338.
- 24
- 24aV. R. Yatham, Y. Shen, R. Martin, Angew. Chem. Int. Ed. 2017, 56, 10915; Angew. Chem. 2017, 129, 11055;
- 24bJ. Hou, A. Ee, H. Cao, H.-W. Ong, J.-H. Xu, J. Wu, Angew. Chem. Int. Ed. 2018, 57, 17220; Angew. Chem. 2018, 130, 17466.