Drawing with Iron on a Gel Containing a Supramolecular Siderophore
Songjun Xiao
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorProf. Paul J. Paukstelis
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Richard D. Ash
Department of Geology, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Peter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorCorresponding Author
Prof. Jeffery T. Davis
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorSongjun Xiao
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorProf. Paul J. Paukstelis
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Richard D. Ash
Department of Geology, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Peter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorCorresponding Author
Prof. Jeffery T. Davis
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorGraphical Abstract
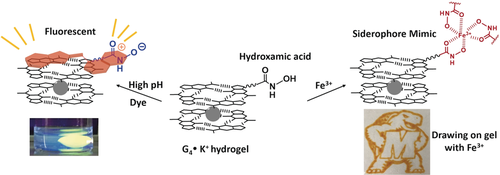
Soft matter, hard shell: pH-responsive hydrogels that can chelate Fe3+ were prepared from guanosine-5′-hydroxamic acid (HA). At high pH, the hydrogel binds thiazole orange, signaled by enhanced fluorescence. The HA units can also act as a supramolecular siderophore to form red complexes with Fe3+, which allowed the patterning of the hydrogel surface with FeCl3.
Abstract
Guanosine-5′-hydroxamic acid (3) forms hydrogels when mixed with guanosine (1) and KCl. The 5′-hydroxamic acid (HA) unit is pH-responsive and also chelates Fe3+. When gels are prepared under basic conditions, the 5′-HA groups are deprotonated and the anionic hydrogel binds cationic thiazole orange (TO), signaled by enhanced fluorescence. The HA nucleoside 3, when immobilized in the G-quartet gel, acts as a supramolecular siderophore to form red complexes with Fe3+. We patterned the hydrogel's surface with FeCl3, by hand and by using a 3D printer. Patterns form instantly, are visible by eye, and can be erased using vitamin C. This hydrogel, combining self-assembled G-quartet and siderophore–Fe3+ motifs, is strong, can be molded into different shapes, and is stable on the bench or under salt water.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201910872-sup-0001-misc_information.pdf2.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. B. Amabilino, D. K. Smith, J. W. Steed, Chem. Soc. Rev. 2017, 46, 2404–2420;
- 1bE. R. Draper, D. J. Adams, Chem 2017, 3, 390–410.
- 2P. R. A. Chivers, D. K. Smith, Nat. Rev. Mater. 2019, 4, 463.
- 3S. Matsumoto, et al., Chem. Eur. J. 2008, 14, 3977–3986.
- 4
- 4aC. Maity, W. E. Hendriksen, J. H. Van Esch, R. Eelkema, Angew. Chem. Int. Ed. 2015, 54, 998–1001; Angew. Chem. 2015, 127, 1012–1015;
- 4bE. R. Draper, E. G. B. Eden, T. O. McDonald, D. J. Adams, Nat. Chem. 2015, 7, 848–852.
- 5For approaches that use non-covalent interactions to control chemical micropatterning:
- 5aY. Wang, S. Oldenhof, F. Versluis, M. Shah, K. Zhang, V. van Steijn, X. Guo, R. Eelkema, J. H. van Esch, Small 2019, 15, 1804154;
- 5bX. Peng, T. Liu, Q. Zhang, C. Shang, Q. W. Bai, H. Wang, Adv. Funct. Mater. 2017, 27, 1701962.
- 6G. M. Peters, J. T. Davis, Chem. Soc. Rev. 2016, 45, 3188–3206.
- 7I. Bang, Biochem. Z. 1910, 26, 293–311.
- 8
- 8aC. Arnal-Hérault, A. Banu, M. Barboiu, M. Michau, A. Van Der Lee, Angew. Chem. Int. Ed. 2007, 46, 4268–4272; Angew. Chem. 2007, 119, 4346–4350;
- 8bB. J. Cafferty, I. Gállego, M. C. Chen, K. I. Farley, R. Eritja, N. V. Hud, J. Am. Chem. Soc. 2013, 135, 2447–2450.
- 9T. Bhattacharyya, Y. P. Kumar, J. Dash, ACS Biomater. Sci. Eng. 2017, 3, 2358–2365.
- 10R. Zhong, et al., Adv. Mater. 2018, 30, 1706887.
- 11A. Biswas, S. Malferrari, D. M. Kalaskar, A. K. Das, Chem. Commun. 2018, 54, 1778–1781.
- 12
- 12aL. E. Buerkle, H. A. von Recum, S. J. Rowan, Chem. Sci. 2012, 3, 564–572;
- 12bA. Rotaru, G. Pricope, T. N. Plank, L. Clima, E. L. Ursu, M. Pinteala, J. T. Davis, M. Barboiu, Chem. Commun. 2017, 53, 12668–12671.
- 13V. Venkatesh, N. K. Mishra, I. Romero-Canelón, R. R. Vernooij, H. Shi, J. P. C. Coverdale, A. Habtemariam, S. Verma, P. J. Sadler, J. Am. Chem. Soc. 2017, 139, 5656–5659.
- 14G. M. Peters, L. P. Skala, T. N. Plank, B. J. Hyman, G. N. Manjunatha Reddy, A. Marsh, S. P. Brown, J. T. Davis, J. Am. Chem. Soc. 2014, 136, 12596–12599.
- 15N. Sreenivasachary, J. M. Lehn, Proc. Natl. Acad. Sci. USA 2005, 102, 5938–5943.
- 16
- 16aS. Xiao, J. T. Davis, Chem. Commun. 2018, 54, 11300–11303;
- 16bS. Xiao, J. T. Davis, Faraday Discuss. 2018, 209, 97–112.
- 17
- 17aR. C. T. Howe, A. P. Smalley, A. P. M. Guttenplan, M. W. R. Doggett, M. D. Eddleston, J. C. Tan, G. O. Lloyd, Chem. Commun. 2013, 49, 4268–4270;
- 17bS. Zhang, et al., Nat. Mater. 2015, 14, 1065–1071.
- 18The crystals were the monohydrate of the monomeric nucleoside G 2⋅H2O. See the Supporting Information for details of the crystal structure. CCDC 1946227 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 19
- 19aB. Monzyk, A. L. Crumbliss, J. Org. Chem. 1980, 45, 4670–4675;
- 19bO. N. Ventura, J. B. Rama, L. Turi, J. J. Dannenberg, J. Am. Chem. Soc. 1993, 115, 5754–5761.
- 20R. Codd, Coord. Chem. Rev. 2008, 252, 1387–1408.
- 21K. Abu-Dari, S. R. Cooper, K. N. Raymond, Inorg. Chem. 1978, 17, 3394–3397.
- 22P. E. Powell, G. R. Cline, C. P. P. Reid, P. J. Szaniszlo, Nature 1980, 287, 833–834.
- 23K. N. Raymond, B. E. Allred, A. K. Sia, Acc. Chem. Res. 2015, 48, 2496–2505.
- 24
- 24aS. R. Cooper, J. V. McArdle, K. N. Raymond, Proc. Natl. Acad. Sci. USA 1978, 75, 3551–3554;
- 24bH. Boukhalfa, A. L. Crumbliss, BioMetals 2002, 15, 325–339.
- 25A. Ponce, L. B. Brostoff, S. K. Gibbons, P. Zavalij, C. Viragh, J. Hooper, S. Alnemrat, K. J. Gaskell, B. Eichhorn, Anal. Chem. 2016, 88, 5152–5158.
- 26Q. Dai, Q. Yu, Y. Tian, X. Xie, A. Song, F. Caruso, J. Hao, J. Cui, ACS Appl. Mater. Interfaces 2019, 11, 29305–29311.
- 27
- 27aS. C. Polomoscanik, C. P. Cannon, T. X. Neenan, S. R. Holmes-Farley, W. H. Mandeville, P. K. Dhal, Biomacromolecules 2005, 6, 2946–2953;
- 27bZ. Liu, Y. Wang, M. Purro, M. P. Xiong, Sci. Rep. 2016, 6, 20923;
- 27cT. Johann, U. Kemmer-Jonas, R. D. Barent, H. Frey, Macromol. Rapid Commun. 2019, 1900282.
- 28V. Setnička, M. Urbanová, K. Volka, S. Nampally, J. M. Lehn, Chem. Eur. J. 2006, 12, 8735–8743.
- 29
- 29aD. Monchaud, C. Allain, H. Bertrand, N. Smargiasso, F. Rosu, V. Gabelica, A. De Cian, J. L. Mergny, M. P. Teulade-Fichou, Biochimie 2008, 90, 1207–1223;
- 29bP. Yang, A. De Cian, M. P. Teulade-Fichou, J. L. Mergny, D. Monchaud, Angew. Chem. Int. Ed. 2009, 48, 2188–2191; Angew. Chem. 2009, 121, 2222–2225.
- 30
- 30aS. Nakayama, I. Kelsey, J. Wang, K. Roelofs, B. Stefane, Y. Luo, V. T. Lee, H. O. Sintim, J. Am. Chem. Soc. 2011, 133, 4856–4864;
- 30bG. M. Peters, L. P. Skala, J. T. Davis, J. Am. Chem. Soc. 2016, 138, 134–139.
- 31Hydrazides have also been suggested as replacements for carboxylic acid groups in pH-responsive hydrogels: B. O. Okesola, D. K. Smith, Chem. Commun. 2013, 49, 11164–11166.
- 32S. Dhungana, J. M. Harrington, P. Gebhardt, U. Möllmann, A. L. Crumbliss, Inorg. Chem. 2007, 46, 8362–8371.
- 33
- 33aM. B. Bannwarth, T. Weidner, E. Eidmann, K. Landfester, D. Crespy, Chem. Mater. 2014, 26, 1300–1302;
- 33bJ. Wang, T. Li, F. Chen, D. Zhou, B. Li, X. Zhou, T. Gan, S. Handschuh-Wang, X. Zhou, Macromol. Rapid Commun. 2018, 39, 1800143.