Stereospecific Synthesis of α-Hydroxy-Cyclopropylboronates from Allylic Epoxides
Laura Amenós
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorDr. Laura Trulli
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorLuis Nóvoa
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorDr. Alejandro Parra
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorCorresponding Author
Dr. Mariola Tortosa
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorLaura Amenós
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorDr. Laura Trulli
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorLuis Nóvoa
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorDr. Alejandro Parra
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorCorresponding Author
Dr. Mariola Tortosa
Organic Chemistry Department, Institute for Advance Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
Search for more papers by this authorGraphical Abstract
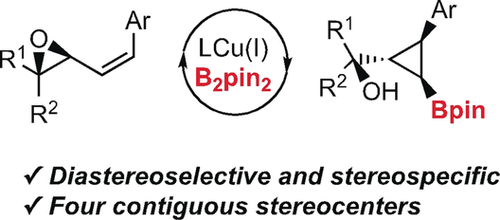
Rigid sp3 scaffolds: A catalytic and stereospecific method for the preparation of enantioenriched α-hydroxy cyclopropylboronates with control in four contiguous stereocenters is described. The reaction involves the borylation of readily available allylic epoxides using an inexpensive CuI salt and a commercially available phosphine ligand. High diastereocontrol is achieved and different diastereomers can be selectively prepared.
Abstract
Herein, we report a catalytic and stereospecific method for the preparation of enantioenriched α-hydroxy cyclopropylboronates with control in four contiguous stereocenters. The reaction involves the borylation of readily available allylic epoxides using an inexpensive Cu(I) salt and a commercially available phosphine ligand. High diastereocontrol is achieved and different diastereomers can be selectively prepared. Functionalization of the carbon–boron bond provides access to different enantiomerically enriched trisubstituted cyclopropanes from a common intermediate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812836-sup-0001-misc_information.pdf6.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Faust, Angew. Chem. Int. Ed. 2001, 40, 2251;
10.1002/1521-3773(20010618)40:12<2251::AID-ANIE2251>3.0.CO;2-R CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2312;
- 1bW. A. Donaldson, Tetrahedron 2001, 57, 8589;
- 1cJ. Pietruszka, Chem. Rev. 2003, 103, 1051;
- 1dL. A. Wessjohann, W. Brandt, T. Thiemann, Chem. Rev. 2003, 103, 1625;
- 1eP. Tang, Y. Qin, Synthesis 2012, 44, 2969;
- 1fD. Y.-K. Chen, R. H. Pouwer, J.-A. Richard, Chem. Soc. Rev. 2012, 41, 4631;
- 1gC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651;
- 1hL. Dian, I. Marek, Chem. Rev. 2018, 118, 8415.
- 2T. T. Talele, J. Med. Chem. 2016, 59, 8712.
- 3R. D. Taylor, M. MacCoss, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845.
- 4For a recent review, see: V. Martín-Heras, A. Parra, M. Tortosa, Synthesis 2018, 50, 470.
- 5D. G. Hall, Boronic Acids, Wiley-VCH, Weinheim, 2005.
10.1002/3527606548 Google Scholar
- 6For an enantioselective approach, see:
- 6aJ. Carreras, A. Caballero, P. J. Pérez, Angew. Chem. Int. Ed. 2018, 57, 2334; For a diastereoselective approach:
- 6bT. Imai, H. Mineta, S. Nishida, J. Org. Chem. 1990, 55, 4986;
- 6cS.-M. Zhou, M.-Z. Deng, L.-J. Xia, M.-H. Tang, Angew. Chem. Int. Ed. 1998, 37, 2845;
10.1002/(SICI)1521-3773(19981102)37:20<2845::AID-ANIE2845>3.0.CO;2-U CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 3061;10.1002/(SICI)1521-3757(19981016)110:20<3061::AID-ANGE3061>3.0.CO;2-A Web of Science® Google Scholar
- 6dJ. E. A. Luithle, J. Pietruszka, A. Witt, Chem. Commun. 1998, 2651;
- 6eJ. E. A. Luithle, J. Pietruszka, J. Org. Chem. 1999, 64, 8287;
- 6fJ. Pietruszka, A. Witt, J. Chem. Soc. Perkin Trans. 1 2000, 4293;
- 6gJ. E. A. Luithle, J. Pietruszka, Eur. J. Org. Chem. 2000, 2557;
- 6hJ. E. A. Luithle, J. Pietruszka, J. Org. Chem. 2000, 65, 9194;
- 6iJ. Pietruszka, A. Witt, W. Frey, Eur. J. Org. Chem. 2003, 3219.
- 7
- 7aM. Rubina, M. Rubin, V. Gevorgyan, J. Am. Chem. Soc. 2003, 125, 7198;
- 7bA. Parra, L. Amenós, M. Guisán-Ceinos, A. López, J. L. García Ruano, M. Tortosa, J. Am. Chem. Soc. 2014, 136, 15833;
- 7cB. Tian, Q. Liu, X. Tong, P. Tian, G.-Q. Lin, Org. Chem. Front. 2014, 1, 1116.
- 8
- 8aH. Ito, Y. Kosaka, K. Nonoyama, Y. Sasaki, M. Sawamura, Angew. Chem. Int. Ed. 2008, 47, 7424; Angew. Chem. 2008, 120, 7534;
- 8bC. Zhong, S. Kunii, Y. Kosaka, M. Sawamura, H. Ito, J. Am. Chem. Soc. 2010, 132, 11440.
- 9For recent diastereoselective methods that do not provide enantiomerically enriched cyclopropylboronates, see:
- 9aM. M. Hussain, H. Li, N. Hussain, M. Ureña, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc. 2009, 131, 6516;
- 9bC. W. Liskey, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 3375;
- 9cS. Miyamura, M. Araki, T. Suzuki, J. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 846; Angew. Chem. 2015, 127, 860;
- 9dJ. He, H. Jiang, R. Takise, R.-Y. Zhu, G. Chen, H.-X. Dai, T. G. Murali Dhar, J. Shi, H. Zhang, P. T. W. Cheng, J.-Q. Yu, Angew. Chem. Int. Ed. 2016, 55, 785; Angew. Chem. 2016, 128, 795;
- 9eG. Benoit, A. B. Charette, J. Am. Chem. Soc. 2017, 139, 1364;
- 9fM. Murai, C. Mizuta, R. Taniguchi, K. Takai, Org. Lett. 2017, 19, 6104;
- 9gM. Sayes, G. Benoit, A. B. Charette, Angew. Chem. Int. Ed. 2018, 57, 13514.
- 10
- 10aJ. Pietruszka, A. Witt, Synlett 2003, 91;
- 10bS. A. Murray, E. C. M. Luc, S. J. Meek, Org. Lett. 2018, 20, 469. We have only considered methods in which the starting hydroxyl vinyl boronates could be prepared in an enantiomerically enriched form.
- 11Reference 9 e reports one example of cyclopropanation with a secondary allylic alcohol (R1 ≠ H). The cyclopropylboronates were obtained as a 1.9:1 mixture of diastereomers.
- 12
- 12aM. Tortosa, Angew. Chem. Int. Ed. 2011, 50, 3950;
- 12bR. Alfaro, A. Parra, J. Alemán, J. L. García Ruano, M. Tortosa, J. Am. Chem. Soc. 2012, 134, 15165;
- 12cC. Jarava-Barrera, A. Parra, A. López, F. Cruz-Acosta, D. Collado-Sanz, D. J. Cárdenas, M. Tortosa, ACS. Catal. 2016, 6, 442;
- 12dM. Guisán-Ceinos, A. Parra, V. Martín-Heras, M. Tortosa, Angew. Chem. Int. Ed. 2016, 55, 6969;
- 12eA. López, T. B. Clark, A. Parra, M. Tortosa, Org. Lett. 2017, 19, 6272;
- 12fC. Jarava-Barrera, A. Parra, L. Amenós, A. Arroyo, M. Tortosa, Chem. Eur. J. 2017, 23, 17478.
- 13
- 13aM. Pineschi, F. Bertolini, V. Di Bussolo, P. Crotti, Curr. Org. Synth. 2009, 6, 290;
- 13bJ. He, J. Ling, P. Chiu, Chem. Rev. 2014, 114, 8037.
- 14A. Berkessel, H. Engler, T. M. Leuther in Science of Synthesis: Catalytic Oxidation in Organic Synthesis, Vol. 1 (Ed.: ), Thieme, Stuttgart, 2017, pp. 245–307.
- 15β-Elimination from alkyl copper species has been previously proposed, see:
- 15aH. Ito, S. Ito, Y. Sasaki, K. Matsuura, M. Sawamura, J. Am. Chem. Soc. 2007, 129, 14856;
- 15bH. Ito, T. Okura, K. Matsuura, M. Sawamura, Angew. Chem. Int. Ed. 2010, 49, 560; Angew. Chem. 2010, 122, 570;
- 15cH. Ohmiya, U. Yokobori, Y. Makida, M. Sawamura, J. Am. Chem. Soc. 2010, 132, 2895;
- 15dY. Shido, M. Yoshida, M. Tanabe, H. Ohmiya, M. Sawamura, J. Am. Chem. Soc. 2012, 134, 18573;
- 15eK. Semba, T. Fujihara, J. Terao, Y. Tsuji, Angew. Chem. Int. Ed. 2013, 52, 12400.
- 16CCDC 1873299 (2 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 17
- 17aS. Harada, N. Kowase, N. Tabuchi, T. Taguchi, Y. Dobashi, A. Dobashi, Y. Hanzawa, Tetrahedron 1998, 54, 753;
- 17bX. Xie, G. Yue, S. Tang, X. Huo, Q. Liang, X. She, X. Pan, Org. Lett. 2005, 7, 4057;
- 17cS. Tang, X. Xie, X. Huo, Q. Liang, X. She, X. Pan, Tetrahedron Lett. 2006, 47, 205;
- 17dR. C. Dhakal, R. K. Dieter, J. Org. Chem. 2013, 78, 12426.
- 18We refer to borylative endo-cyclization to the one in which the boryl moiety is directly attached to the ring formed. On the other hand, borylative exo-cyclization is that in which the boryl moiety is not directly attached to the ring formed.

- 19For copper-catalyzed borylative exo-cyclizations of alkenes, see:
- 19aH. Ito, T. Toyoda, M. Sawamura, J. Am. Chem. Soc. 2010, 132, 5990;
- 19bK. Kubota, E. Yamamoto, H. Ito, J. Am. Chem. Soc. 2013, 135, 2635;
- 19cJ. Royes, S. Ni, A. Farré, E. La Cascia, J. J. Carbó, A. B. Cuenca, F. Maseras, E. Fernández, ACS Catal. 2018, 8, 2833;
- 19dFor a copper(I)-catalyzed borylative radical cyclization, see: H. Iwamoto, S. Akiyama, K. Hayama, H. Ito, Org. Lett. 2017, 19, 2614.
- 20
- 20aA. Hirai, A. Matsui, K. Komatsu, K. Tanino, M. Miyashita, Chem. Commun. 2002, 1970;
- 20bM. Pineschi, N. J. Chem. 2004, 28, 657;
- 20cR. K. Dieter, Y. Huang, F. Guo, J. Org. Chem. 2012, 77, 4949.
- 21CCDC 1873303 (2 j) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 22The electron deficient aryl unit probably favors the formation of an extended copper-enolate that could cyclize to give 2 j, leaving Hb cis to the boryl moiety to minimize steric effects. Although a rapid isomerizacion of the double bond of the allylic epoxide cannot be ruled out, we did not detect any E-allylic epoxide when we monitored the reaction by 1H NMR.
 For the formation of a related extended copper-enolate from a benzylic intermediate, see: J. Lee, S. Radomkit, S. Torker, J. del Pozo, A. H. Hoveyda, Nat. Chem. 2018, 10, 99.
For the formation of a related extended copper-enolate from a benzylic intermediate, see: J. Lee, S. Radomkit, S. Torker, J. del Pozo, A. H. Hoveyda, Nat. Chem. 2018, 10, 99.
- 23The relative configuration of 2 k was by established by comparison of the 1H NMR data with compound 2 j.
- 24The enantiomeric ratio of the products is expected to be the same as the allylic epoxides, since one of the stereocenters in the oxirane ring is not modified through the transformation. Nevertheless, we proved that the enantiomeric ratio of epoxide 1 m was preserved in cyclopropylboronate 2 m. See the Supporting Information for details.
- 25
- 25aW. Pei, I. J. Krauss, J. Am. Chem. Soc. 2011, 133, 18514;
- 25bH. Lin, W. Pei, H. Wang, K. N. Houk, I. J. Krauss, J. Am. Chem. Soc. 2013, 135, 82;
- 25cH. Lin, L. Tian, I. J. Krauss, J. Am. Chem. Soc. 2015, 137, 13176.
- 26S. Norsikian, I. Marek, J.-F. Poisson, J. F. Normant, J. Org. Chem. 1997, 62, 4898.
- 27Compound E provides cyclopropylboronate 2 after hydrolysis of the O−B bond during work-up.